Brain glucose metabolism and nigrostriatal degeneration in isolated rapid eye movement sleep behaviour disorder
- PMID: 36844148
- PMCID: PMC9945851
- DOI: 10.1093/braincomms/fcad021
Brain glucose metabolism and nigrostriatal degeneration in isolated rapid eye movement sleep behaviour disorder
Abstract
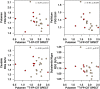
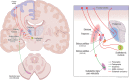
Alterations of cerebral glucose metabolism can be detected in patients with isolated rapid eye movement sleep behaviour disorder, a prodromal feature of neurodegenerative diseases with α-synuclein pathology. However, metabolic characteristics that determine clinical progression in isolated rapid eye movement sleep behaviour disorder and their association with other biomarkers need to be elucidated. We investigated the pattern of cerebral glucose metabolism on 18F-fluorodeoxyglucose PET in patients with isolated rapid eye movement sleep behaviour disorder, differentiating between those who clinically progressed and those who remained stable over time. Second, we studied the association between 18F-fluorodeoxyglucose PET and lower dopamine transporter availability in the putamen, another hallmark of synucleinopathies. Patients with isolated rapid eye movement sleep behaviour disorder from the Mayo Clinic Alzheimer's Disease Research Center and Center for Sleep Medicine (n = 22) and age-and sex-matched clinically unimpaired controls (clinically unimpaired; n = 44) from the Mayo Clinic Study of Aging were included. All participants underwent 18F-fluorodeoxyglucose PET and dopamine transporter imaging with iodine 123-radiolabeled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane on single-photon emission computerized tomography. A subset of patients with isolated rapid eye movement sleep behaviour disorder with follow-up evaluations (n = 17) was classified as isolated rapid eye movement sleep behaviour disorder progressors (n = 7) if they developed mild cognitive impairment or Parkinson's disease; or isolated rapid eye movement sleep behaviour disorder stables (n = 10) if they remained with a diagnosis of isolated rapid eye movement sleep behaviour disorder with no cognitive impairment. Glucose metabolic abnormalities in isolated rapid eye movement sleep behaviour disorder were determined by comparing atlas-based regional 18F-fluorodeoxyglucose PET uptake between isolated rapid eye movement sleep behaviour disorder and clinically unimpaired. Associations between 18F-fluorodeoxyglucose PET and dopamine transporter availability in the putamen were analyzed with Pearson's correlation within the nigrostriatal pathway structures and with voxel-based analysis in the cortex. Patients with isolated rapid eye movement sleep behaviour disorder had lower glucose metabolism in the substantia nigra, retrosplenial cortex, angular cortex, and thalamus, and higher metabolism in the amygdala and entorhinal cortex compared with clinically unimpaired. Patients with isolated rapid eye movement sleep behaviour disorder who clinically progressed over time were characterized by higher glucose metabolism in the amygdala and entorhinal cortex, and lower glucose metabolism in the cerebellum compared with clinically unimpaired. Lower dopamine transporter availability in the putamen was associated with higher glucose metabolism in the pallidum within the nigrostriatal pathway; and with higher 18F-fluorodeoxyglucose uptake in the amygdala, insula, and temporal pole on a voxel-based analysis, although these associations did not survive after correcting for multiple comparisons. Our findings suggest that cerebral glucose metabolism in isolated rapid eye movement sleep behaviour disorder is characterized by hypometabolism in regions frequently affected during the prodromal stage of synucleinopathies, potentially reflecting synaptic dysfunction. Hypermetabolism is also seen in isolated rapid eye movement sleep behaviour disorder, suggesting that synaptic metabolic disruptions may be leading to a lack of inhibition, compensatory mechanisms, or microglial activation, especially in regions associated with nigrostriatal degeneration.
Keywords: FDG; Lewy bodies disease; PET; SPECT; isolated REM sleep behaviour disorder.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
Interrelation of striatal dopamine, brain metabolism and cognition in dementia with Lewy bodies.Brain. 2022 Dec 19;145(12):4448-4458. doi: 10.1093/brain/awac084. Brain. 2022. PMID: 35234856
-
Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study.Lancet Neurol. 2011 Sep;10(9):797-805. doi: 10.1016/S1474-4422(11)70152-1. Epub 2011 Jul 28. Lancet Neurol. 2011. PMID: 21802993
-
Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected].Lancet Neurol. 2010 Nov;9(11):1070-7. doi: 10.1016/S1474-4422(10)70216-7. Epub 2010 Sep 16. Lancet Neurol. 2010. PMID: 20846908
-
Circuit imaging biomarkers in preclinical and prodromal Parkinson's disease.Mol Med. 2021 Sep 16;27(1):111. doi: 10.1186/s10020-021-00327-x. Mol Med. 2021. PMID: 34530732 Free PMC article. Review.
-
Correlations of Neuropsychological and Metabolic Brain Changes in Parkinson's Disease and Other α-Synucleinopathies.Front Neurol. 2019 Nov 14;10:1204. doi: 10.3389/fneur.2019.01204. eCollection 2019. Front Neurol. 2019. PMID: 31798525 Free PMC article. Review.
Cited by
-
Longitudinal FDG-PET Metabolic Change Along the Lewy Body Continuum.JAMA Neurol. 2025 Mar 1;82(3):285-294. doi: 10.1001/jamaneurol.2024.4643. JAMA Neurol. 2025. PMID: 39804619
-
Neuroimaging biomarkers in the biological definition of Parkinson's disease and dementia with Lewy bodies - EANM position on current state, unmet needs and future perspectives.Eur J Nucl Med Mol Imaging. 2024 Oct;51(12):3496-3500. doi: 10.1007/s00259-024-06803-w. Eur J Nucl Med Mol Imaging. 2024. PMID: 38907856 Free PMC article. No abstract available.
-
Operationalizing postmortem pathology-MRI association studies in Alzheimer's disease and related disorders with MRI-guided histology sampling.Acta Neuropathol Commun. 2025 May 28;13(1):120. doi: 10.1186/s40478-025-02030-y. Acta Neuropathol Commun. 2025. PMID: 40437594 Free PMC article.
-
Implications and opportunities regarding biological frameworks in overt and prodromal dementia with Lewy bodies.Alzheimers Dement. 2025 Jul;21(7):e70470. doi: 10.1002/alz.70470. Alzheimers Dement. 2025. PMID: 40684248 Free PMC article. Review.
-
Choroid plexus enlargement in patients with rapid eye movement sleep behavior disorder: relevance to glymphatic system dysfunction.Sleep Biol Rhythms. 2025 Jan 6;23(2):189-195. doi: 10.1007/s41105-024-00568-3. eCollection 2025 Apr. Sleep Biol Rhythms. 2025. PMID: 40190611
References
-
- Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: A new category of parasomnia. Sleep. 2002;25(2):293–308. - PubMed
-
- Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: Demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(Pt 2):331–339. - PubMed
-
- Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622–630. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
