Metal-Free Synthesis of Functionalized Quinolines from 2-Styrylanilines and 2-Methylbenzothiazoles/2-Methylquinolines
- PMID: 36844512
- PMCID: PMC9948197
- DOI: 10.1021/acsomega.2c07736
Metal-Free Synthesis of Functionalized Quinolines from 2-Styrylanilines and 2-Methylbenzothiazoles/2-Methylquinolines
Abstract
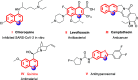
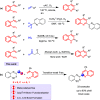
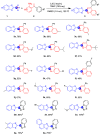
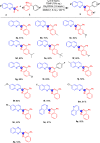
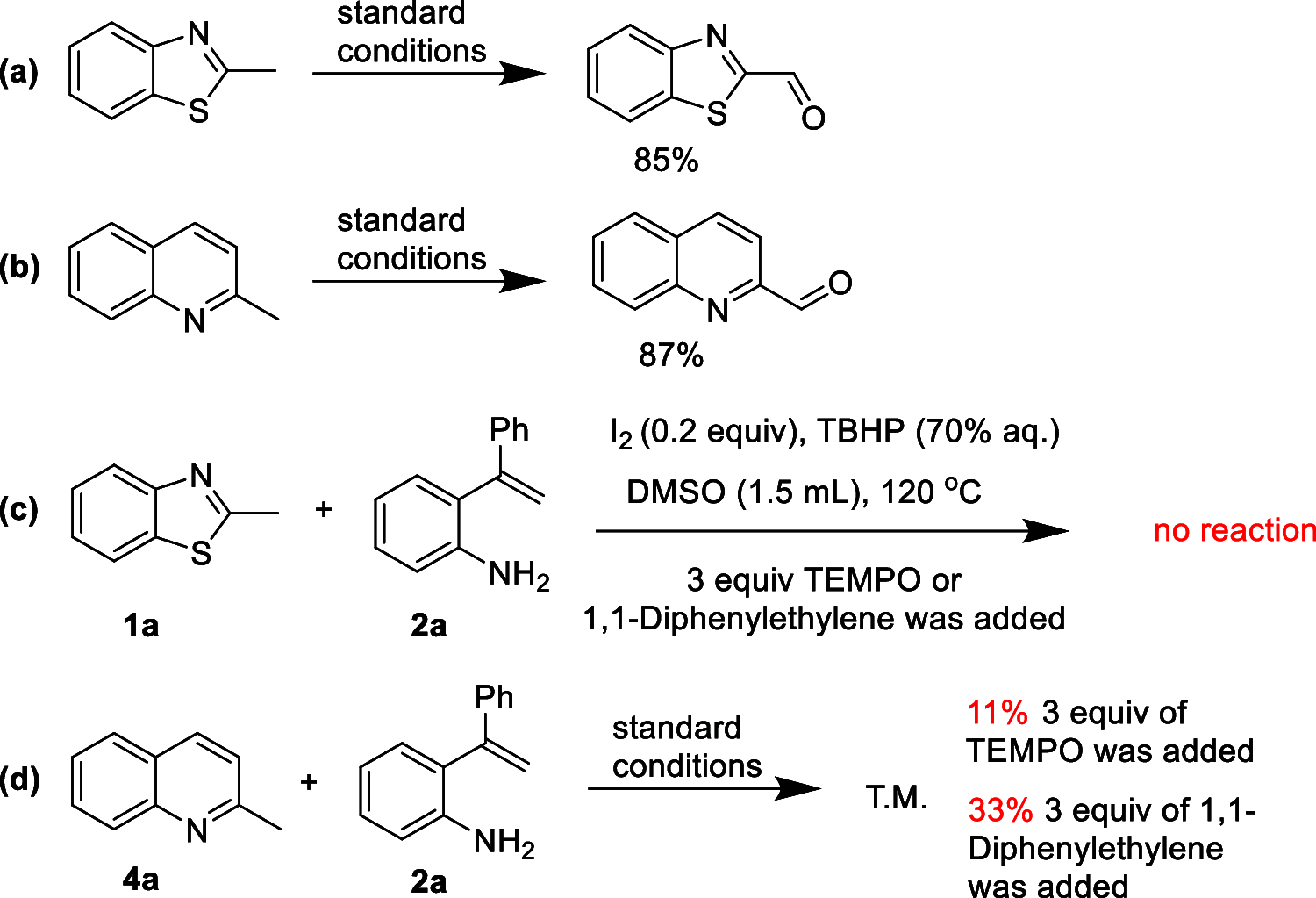
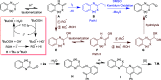
A facile functionalization of C(sp3)-H bonds and tandem cyclization strategy to synthesize quinoline derivatives from 2-methylbenzothiazoles or 2-methylquinolines and 2-styrylanilines has been developed. This work avoids the requirement for transition metals, offering a mild approach to activation of C(sp3)-H bonds and formation of new C-C and C-N bonds. This strategy features excellent functional group tolerance and scaled-up synthetic capability, thus providing an efficient and environmentally friendly access to medicinally valuable quinolines.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
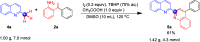
Similar articles
-
Transition Metal-Catalyzed C-H Activation/Functionalization of 8-Methylquinolines.Chem Rec. 2024 Nov;24(11):e202400116. doi: 10.1002/tcr.202400116. Epub 2024 Oct 18. Chem Rec. 2024. PMID: 39422078 Review.
-
C(sp3)-H Bond Functionalization of 8-Methylquinolines.Chem Asian J. 2025 Mar 3;20(5):e202401266. doi: 10.1002/asia.202401266. Epub 2025 Feb 6. Chem Asian J. 2025. PMID: 39736085 Review.
-
Metal-Free Photochemical Radical Cyclization for the Synthesis of Pyrrolo[3,2-c]-quinolines with One-Carbon Unit.Chem Asian J. 2022 Dec 14;17(24):e202200963. doi: 10.1002/asia.202200963. Epub 2022 Nov 24. Chem Asian J. 2022. PMID: 36332196
-
Rh(iii)-catalyzed sp3/sp2-C-H heteroarylations via cascade C-H activation and cyclization.Chem Sci. 2024 Mar 27;15(17):6544-6551. doi: 10.1039/d3sc06955a. eCollection 2024 May 1. Chem Sci. 2024. PMID: 38699273 Free PMC article.
-
A Four-Component Reaction for the Synthesis of β-Quinoline Allylic Sulfones under Iron Catalysis.J Org Chem. 2018 Sep 7;83(17):10420-10429. doi: 10.1021/acs.joc.8b01486. Epub 2018 Aug 14. J Org Chem. 2018. PMID: 30073837
References
-
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. - DOI - PubMed
- Heravi M. M.; Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020, 10, 44247–44311. 10.1039/D0RA09198G. - DOI - PMC - PubMed
- Joule J. A. Natural products containing nitrogen heterocycles-some highlights 1990-2015. Adv. Heterocycl. Chem. 2016, 119, 81–106. 10.1016/bs.aihch.2015.10.005. - DOI
- Gao B.; Yang B.; Feng X.; Li C. Recent advances in the biosynthesis strategies of nitrogen heterocyclic natural products. Nat. Prod. Rep. 2022, 39, 139–162. 10.1039/D1NP00017A. - DOI - PubMed
- Haiduc I. Review Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 1. Five-membered and smaller rings. J. Coord. Chem. 2019, 72, 2127–2159. 10.1080/00958972.2019.1641702. - DOI
-
- Shang X. F.; Morris-Natschke S. L.; Liu Y. Q.; Guo X.; Xu X. S.; Goto M.; Li J. C.; Yang G. Z.; Lee K. H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. 10.1002/med.21466. - DOI - PMC - PubMed
- Ajani O. O.; Iyaye K. T.; Ademosun O. T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs - a review. RSC Adv. 2022, 12, 18594–18614. 10.1039/D2RA02896D. - DOI - PMC - PubMed
- Michael J. P. Quinoline, quinazoline, and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. 10.1039/B612168N. - DOI - PubMed
- Lihumis H. S.; Alameri A. A.; Zaooli R. H. A review on recent development and biological applications of benzothiazole derivatives. Prog. Chem. Biochem. Res. 2022, 5, 147–164. 10.22034/pcbr.2022.330703.1214. - DOI
- Moor L. F. E.; Vasconcelos T. R. A.; da R Reis R.; Pinto L. S. S.; Da Costa T. M. Quinoline: An Attractive Scaffold in Drug Design. Mini-Rev. Med. Chem. 2021, 21, 2209–2226. 10.2174/1389557521666210210155908. - DOI - PubMed
LinkOut - more resources
Full Text Sources

