Discrete Singular Metallophilic Interaction in Stable Large 12-Membered Binuclear Silver and Gold Metallamacrocycles of Amido-Functionalized Imidazole and 1,2,4-Triazole-Derived N-Heterocyclic Carbenes
- PMID: 36844527
- PMCID: PMC9947987
- DOI: 10.1021/acsomega.2c06729
Discrete Singular Metallophilic Interaction in Stable Large 12-Membered Binuclear Silver and Gold Metallamacrocycles of Amido-Functionalized Imidazole and 1,2,4-Triazole-Derived N-Heterocyclic Carbenes
Abstract
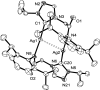
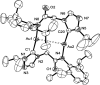
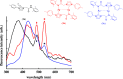
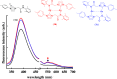
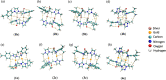
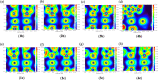
Metallophilic interactions were observed in four pairs of 12-membered metallamacrocyclic silver and gold complexes of imidazole-derived N-heterocyclic carbenes (NHCs), [1-(R1)-3-N-(2,6-di-(R2)-phenylacetamido)-imidazol-2-ylidene]2M2 [R1 = p-MeC6H4, R2 = Me, M = Ag (1b) and Au (1c); R1 = Me, R2 = i-Pr, M = Ag (2b) and Au (2c); R1 = Et, R2 = i-Pr, M = Ag (3b) and Au (3c)], and a 1,2,4-triazole-derived N-heterocyclic carbene (NHC), [1-(i-Pr)-4-N-(2,6-di-(i-Pr)-phenylacetamido)-1,2,4-triazol-2-ylidene]2M2 [M = Ag (4b) and Au (4c)]. The X-ray diffraction, photoluminescence, and computational studies indicate the presence of metallophilic interactions in these complexes, which are significantly influenced by the sterics and the electronics of the N-amido substituents of the NHC ligands. The argentophilic interaction in the silver 1b-4b complexes was stronger than the aurophilic interaction in the gold 1c-4c complexes, with the metallophilic interaction decreasing in the order 4b > 1b > 1c > 4c > 3b > 3c > 2b > 2c. The 1b-4b complexes were synthesized from the corresponding amido-functionalized imidazolium chloride 1a-3a and the 1,2,4-triazolium chloride 4a salts upon treatment with Ag2O. The reaction of 1b-4b complexes with (Me2S)AuCl gave the gold 1c-4c complexes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
From large 12-membered macrometallacycles to ionic (NHC)2M+Cl- type complexes of gold and silver by modulation of the N-substituent of amido-functionalized N-heterocyclic carbene (NHC) ligands.Inorg Chem. 2008 May 19;47(10):4153-65. doi: 10.1021/ic702186g. Epub 2008 Apr 15. Inorg Chem. 2008. PMID: 18410089
-
Highly convenient regioselective intermolecular hydroamination of alkynes yielding ketimines catalyzed by gold(I) complexes of 1,2,4-triazole based N-heterocyclic carbenes.Inorg Chem. 2010 Jun 7;49(11):4972-83. doi: 10.1021/ic100087d. Inorg Chem. 2010. PMID: 20429537
-
Shorter argentophilic interaction than aurophilic interaction in a pair of dimeric {(NHC)MCl}(2) (M = Ag, Au) complexes supported over a N/O-functionalized N-heterocyclic carbene (NHC) ligand.Inorg Chem. 2008 Jan 7;47(1):230-40. doi: 10.1021/ic701830m. Epub 2007 Dec 12. Inorg Chem. 2008. PMID: 18072766
-
Luminescent di- and polynuclear organometallic gold(I)-metal (Au2, {Au2Ag}n and {Au2Cu}n) compounds containing bidentate phosphanes as active antimicrobial agents.Chemistry. 2012 Mar 19;18(12):3659-74. doi: 10.1002/chem.201103145. Epub 2012 Feb 14. Chemistry. 2012. PMID: 22334444 Free PMC article.
-
Ag-NHC Complexes in the π-Activation of Alkynes.Molecules. 2023 Jan 18;28(3):950. doi: 10.3390/molecules28030950. Molecules. 2023. PMID: 36770617 Free PMC article. Review.
References
-
- Sculfort S.; Braunstein P. Intramolecular d10–d10 interactions in heterometallic clusters of the transition metals. Chem. Soc. Rev. 2011, 40, 2741–2760. 10.1039/C0CS00102C. - DOI - PubMed
- Pyykkö P. Theoretical Chemistry of Gold. Angew. Chem., Int. Ed. 2004, 43, 4412–4456. 10.1002/anie.200300624. - DOI - PubMed
-
- Vijayaraghavan R. K.; Chandran D.; Vijayaraghavan R. K.; McCoy A. P.; Daniels S.; McNally P. J. Highly enhanced UV responsive conductivity and blue emission in transparent CuBr films: implication for emitter and dosimeter applications. J. Mater. Chem. C 2017, 5, 10270–10279. 10.1039/C7TC02838E. - DOI
- Kishimura A.; Yamashita T.; Yamaguchi K.; Aida T. Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater. 2005, 4, 546–549. 10.1038/nmat1401. - DOI - PubMed
-
- Henkelis J. J.; Barnett S. A.; Harding L. P.; Hardie M. J. Coordination Polymers Utilizing N-Oxide Functionalized Host Ligands. Inorg. Chem. 2012, 51, 10657–10674. 10.1021/ic300940k. - DOI - PubMed
- Serpe A.; Artizzu F.; Marchiò L.; Mercuri M. L.; Pilia L.; Deplano P. Argentophilic Interactions in Mono-, Di-, and Polymeric Ag(I) Complexes with N,N′-Dimethyl-piperazine-2,3-dithione and Iodide. Cryst. Growth Des. 2011, 11, 1278–1286. 10.1021/cg1015065. - DOI
- Bayler A.; Schier A.; Bowmaker G. A.; Schmidbaur H. Gold Is Smaller than Silver. Crystal Structures of [Bis(trimesitylphosphine)gold(I)] and [Bis(trimesitylphosphine)silver(I)] Tetrafluoroborate. J. Am. Chem. Soc. 1996, 118, 7006–7007. 10.1021/ja961363v. - DOI
-
- Gil-Rubio J.; Vicente J. The Coordination and Supramolecular Chemistry of Gold Metalloligands. Chem. – Eur. J. 2018, 24, 32–46. 10.1002/chem.201703574. - DOI - PubMed
- Yam V. W.-W.; Wong K. M.-C. Luminescent metal complexes of d6, d8 and d10 transition metal centres. Chem. Commun. 2011, 47, 11579–11592. 10.1039/C1CC13767K. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

