Vanillin-Based Indolin-2-one Derivative Bearing a Pyridyl Moiety as a Promising Anti-Breast Cancer Agent via Anti-Estrogenic Activity
- PMID: 36844536
- PMCID: PMC9948168
- DOI: 10.1021/acsomega.2c07793
Vanillin-Based Indolin-2-one Derivative Bearing a Pyridyl Moiety as a Promising Anti-Breast Cancer Agent via Anti-Estrogenic Activity
Abstract
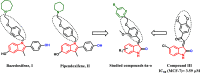
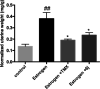
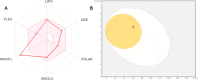
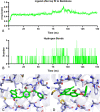
The structure-based design introduced indoles as an essential motif in designing new selective estrogen receptor modulators employed for treating breast cancer. Therefore, here, a series of synthesized vanillin-substituted indolin-2-ones were screened against the NCI-60 cancer cell panel followed by in vivo, in vitro, and in silico studies. Physicochemical parameters were evaluated with HPLC and SwissADME tools. The compounds demonstrated promising anti-cancer activity for the MCF-7 breast cancer cell line (GI = 6-63%). The compound with the highest activity (6j) was selective for the MCF-7 breast cancer cell line (IC50 = 17.01 μM) with no effect on the MCF-12A normal breast cell line supported by real-time cell analysis. A morphological examination of the used cell lines confirmed a cytostatic effect of compound 6j. It inhibited both in vivo and in vitro estrogenic activity, triggering a 38% reduction in uterine weight induced by estrogen in an immature rat model and hindering 62% of ER-α receptors in in vitro settings. In silico molecular docking and molecular dynamics simulation studies supported the stability of the ER-α and compound 6j protein-ligand complex. Herein, we report that indolin-2-one derivative 6j is a promising lead compound for further pharmaceutical formulations as a potential anti-breast cancer drug.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Bender O.; Atalay A.. Polyphenol Chlorogenic Acid, Antioxidant Profile, and Breast Cancer. In Cancer; Elsevier, 2021; pp 311–321.
-
- Fisher B.; Costantino J. P.; Wickerham D. L.; Redmond C. K.; Kavanah M.; Cronin W. M.; Vogel V.; Robidoux A.; Dimitrov N.; Atkins J.; Daly M.; Wieand S.; Tan-Chiu E.; Ford L.; Wolmark N.; Breast other N. S. A.; Investigators B. P. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. 10.1093/jnci/90.18.1371. - DOI - PubMed
LinkOut - more resources
Full Text Sources

