Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective
- PMID: 36844550
- PMCID: PMC9948191
- DOI: 10.1021/acsomega.2c06843
Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective
Abstract
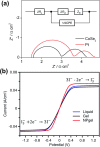
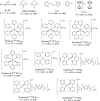
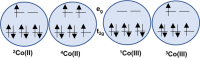
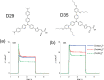
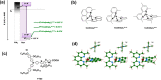
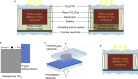
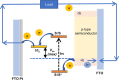
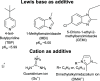
A redox electrolyte is a crucial part of dye-sensitized solar cells (DSSCs), which plays a significant role in the photovoltage and photocurrent of the DSSCs through efficient dye regeneration and minimization of charge recombination. An I-/I3 - redox shuttle has been mostly utilized, but it limits the open-circuit voltage (V oc) to 0.7-0.8 V. To improve the V oc value, an alternative redox shuttle with more positive redox potential is required. Thus, by utilizing cobalt complexes with polypyridyl ligands, a significant power conversion efficiency (PCE) of above 14% with a high V oc of up to 1 V under 1-sun illumination was achieved. Recently, the V oc of a DSSC has exceeded 1 V with a PCE of around 15% by using Cu-complex-based redox shuttles. The PCE of over 34% in DSSCs under ambient light by using these Cu-complex-based redox shuttles also proves the potential for the commercialization of DSSCs in indoor applications. However, most of the developed highly efficient porphyrin and organic dyes cannot be used for the Cu-complex-based redox shuttles due to their higher positive redox potentials. Therefore, the replacement of suitable ligands in Cu complexes or an alternative redox shuttle with a redox potential of 0.45-0.65 V has been required to utilize the highly efficient porphyrin and organic dyes. As a consequence, for the first time, the proposed strategy for a PCE enhancement of over 16% in DSSCs with a suitable redox shuttle is made by finding a superior counter electrode to enhance the fill factor and a suitable near-infrared (NIR)-absorbing dye for cosensitization with the existing dyes to further broaden the light absorption and enhance the short-circuit current density (J sc) value. This review comprehensively analyzes the redox shuttles and redox-shuttle-based liquid electrolytes for DSSCs and gives recent progress and perspectives.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Perez R.; Zweibel K.; Hoff T. E. Solar Power Generation in the US: Too Expensive, or a Bargain?. Energy Policy 2011, 39 (11), 7290–7297. 10.1016/j.enpol.2011.08.052. - DOI
-
- Launch of new RICOH EH DSSC modules with 20% increase in power generation | Global | Ricoh. https://www.ricoh.com/release/2021/0513_1/ (accessed 2021-05-31).
-
- News - Exeger. https://www.exeger.com/news/ (accessed 2021-08-11).
-
- Kang S. H.; Jeong M. J.; Eom Y. K.; Choi I. T.; Kwon S. M.; Yoo Y.; Kim J.; Kwon J.; Park J. H.; Kim H. K. Porphyrin Sensitizers with Donor Structural Engineering for Superior Performance Dye-Sensitized Solar Cells and Tandem Solar Cells for Water Splitting Applications. Adv. Energy Mater. 2017, 7 (7), 1602117. 10.1002/aenm.201602117. - DOI
-
- Eom Y. K.; Kang S. H.; Choi I. T.; Yoo Y.; Kim J.; Kim H. K. Significant Light Absorption Enhancement by a Single Heterocyclic Unit Change in the π-Bridge Moiety from Thieno[3,2-b]Benzothiophene to Thieno[3,2-b]Indole for High Performance Dye-Sensitized and Tandem Solar Cells. J. Mater. Chem. A 2017, 5 (5), 2297–2308. 10.1039/C6TA09836C. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

