Design, Synthesis, and Biological Evaluation Studies of Novel Naphthalene-Chalcone Hybrids As Antimicrobial, Anticandidal, Anticancer, and VEGFR-2 Inhibitors
- PMID: 36844559
- PMCID: PMC9947975
- DOI: 10.1021/acsomega.2c07256
Design, Synthesis, and Biological Evaluation Studies of Novel Naphthalene-Chalcone Hybrids As Antimicrobial, Anticandidal, Anticancer, and VEGFR-2 Inhibitors
Abstract
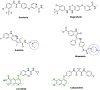
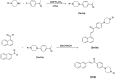
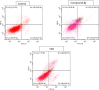
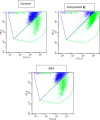
Cancer is a progressive disease that is frequently encountered worldwide. The incidence of cancer is increasing with the changing living conditions around the world. The side-effect profile of existing drugs and the resistance developing in long-term use increase the need for novel drugs. In addition, cancer patients are not resistant to bacterial and fungal infections due to the suppression of the immune system during the treatment. Rather than adding a new antibacterial or antifungal drug to the current treatment plan, the fact that the drug with anticancer activity has these effects (antibacterial and antifungal) will increase the patient's quality of life. For this purpose, in this study, a series of 10 new naphthalene-chalcone derivatives were synthesized and their anticancer-antibacterial-antifungal properties were investigated. Among the compounds, compound 2j showed activity against the A549 cell line with an IC50 = 7.835 ± 0.598 μM. This compound also has antibacterial and antifungal activity. The apoptotic potential of the compound was measured by flow cytometry and showed apoptotic activity of 14.230%. The compound also showed 58.870% mitochondrial membrane potential. Compound 2j inhibited VEGFR-2 enzyme with IC50 = 0.098 ± 0.005 μM. Molecular docking studies of the compounds were carried out by in silico methods against VEGFR-2 and caspase-3 enzymes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
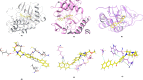
References
-
- El Ghalia H.; Amina G.; El Aissouq A.; Oussama C.; Hicham E. H.; Abdelkrim O.; Mohammed B. A quantitative study of the structure-activity relationship and molecular docking of 5.6. 7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents using quantum chemical descriptors and statistical methods. J. Mol. Struct. 2022, 1270, 133794. 10.1016/j.molstruc.2022.133794. - DOI
-
- Qi B.; Wang F.; He H.; Fan M.; Hu L.; Xiong L.; Gong G.; Shi S.; Song X. Identification of (S)-1-(2-(2, 4-difluorophenyl)-4-oxothiazolidin-3-yl)-3-(4-((7-(3-(4-ethylpiperazin-1-yl) propoxy)-6-methoxyquinolin-4-yl) oxy)-3, 5-difluorophenyl) urea as a potential anti-colorectal cancer agent. Eur. J. Med. Chem. 2022, 239, 114561. 10.1016/j.ejmech.2022.114561. - DOI - PubMed
-
- JawalePatil P. D.; Bhamidipati K.; Damale M. G.; Sangshetti J. N.; Puvvada N.; Bhosale R. S.; Ingle R. D.; Pawar R. P.; Bhosale S. V.; Bhosale S. V. Synthesis of naphthalimide derivatives bearing benzothiazole and thiazole moieties: In vitro anticancer and in silico ADMET study. J. Mol. Struct. 2022, 1263, 133173. 10.1016/j.molstruc.2022.133173. - DOI
-
- Pérez-Soto M.; Peñalver P.; Street S. T.; Weenink D.; O’Hagan M. P.; Ramos-Soriano J.; Jiang Y. J.; Hollingworth G. J.; Galan M. C.; Morales J. C. Structure-activity relationship studies on divalent naphthalene diimide G quadruplex ligands with anticancer and antiparasitic activity. Bioorg. Med. Chem. 2022, 71, 116946. 10.1016/j.bmc.2022.116946. - DOI - PubMed
- Wang X.; Lu Y.; Sun D.; Qian J.; Tu S.; Yue W.; Lin H.; Tang H.; Meng F.; He Q.; et al. Discovery of 4-methoxy-N-(1-naphthyl) benzenesulfonamide derivatives as small molecule dual-target inhibitors of tubulin and signal transducer and activator of transcription 3 (STAT3) based on ABT-751. Bioorg. Chem. 2022, 125, 105864. 10.1016/j.bioorg.2022.105864. - DOI - PubMed
- Gurung S. K.; Kumari S.; Dana S.; Mandal K.; Sen S.; Mukhopadhyay P.; Mondal N. DNA damage, cell cycle perturbation and cell death by naphthalene diimide derivative in gastric cancer cells. Chem-Bio. Interact. 2022, 358, 109881. 10.1016/j.cbi.2022.109881. - DOI - PubMed
- Husseiny E.; El menofy N.; El-Sebaey S. 1,8-Diaminonaphthalene-derived pharmacophore as potent anti-MRSA with dual DNA gyrase and topoisomerase IV inhibition. Egypt. J. Chem. 2021, 65 (3), 1–17. 10.21608/ejchem.2021.104410.4824. - DOI
- Alorini T. A.; Al-Hakimi A. N.; El-Sayed Saeed S.; Alhamzi E. H. L.; Albadri A. E. A. E. Synthesis, characterization, and anticancer activity of some metal complexes with a new Schiff base ligand. Arab. J. Chem. 2022, 15 (2), 103559. 10.1016/j.arabjc.2021.103559. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

