Synthesis of α-CF3 Amides via Palladium-Catalyzed Carbonylation of 2-Bromo-3,3,3-trifluoropropene
- PMID: 36844566
- PMCID: PMC9948557
- DOI: 10.1021/acsomega.2c08206
Synthesis of α-CF3 Amides via Palladium-Catalyzed Carbonylation of 2-Bromo-3,3,3-trifluoropropene
Abstract
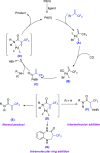
Amide compounds are important organic compounds, which play an important role in biomedical chemistry, materials science, life science, and other fields. The synthesis of α-CF3 amides, especially compounds containing 3-(trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepine-2-one, has long been a challenge due to the tensile properties and instability of the rings. Here, we report an example of palladium-catalyzed carbonylation of CF3-containing olefin to form α-CF3 acrylamide. By controlling the ligands, we can get different amide compounds as products. This method has good substrate adaptability and functional group tolerance.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Palladium Catalyzed Cascade Azidation/Carbonylation of Aryl Halides with Sodium Azide for the Synthesis of Amides.Chem Asian J. 2021 Mar 1;16(5):503-506. doi: 10.1002/asia.202001463. Epub 2021 Feb 2. Chem Asian J. 2021. PMID: 33470007
-
Highly stereoselective synthesis of (E)- and (Z)-alpha-fluoro-alpha,beta-unsaturated esters and (E)- and (Z)-alpha-fluoro-alpha,beta-unsaturated amides from 1-bromo-1-fluoroalkenes via palladium-catalyzed carbonylation reactions.J Org Chem. 2005 May 27;70(11):4346-53. doi: 10.1021/jo050314a. J Org Chem. 2005. PMID: 15903310
-
Palladium-catalyzed approach to primary amides using nongaseous precursors.Org Lett. 2011 Aug 19;13(16):4454-7. doi: 10.1021/ol201808y. Epub 2011 Jul 26. Org Lett. 2011. PMID: 21790124
-
A Brief Review on the Synthesis of the N-CF3 Motif in Heterocycles.Molecules. 2023 Mar 28;28(7):3012. doi: 10.3390/molecules28073012. Molecules. 2023. PMID: 37049775 Free PMC article. Review.
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
Cited by
-
Copper-Catalyzed Synthesis of 4-CF3-1,2,3-Triazoles: An Efficient and Facile Approach via Click Reaction.Molecules. 2024 Mar 7;29(6):1191. doi: 10.3390/molecules29061191. Molecules. 2024. PMID: 38542827 Free PMC article.
References
-
- Kirsch P.Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2004.
- Mikami K.; Itoh Y.; Yamanaka M. Fluorinated Carbonyl and Olefinic Compounds: Basic Character and Asymmetric Catalytic Reactions. Chem. Rev. 2004, 104, 1–16. 10.1021/cr030685w. - DOI - PubMed
- Begue J. P.; Bonnet-Delpon D.. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, 2008.
- Hagmann W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. 10.1021/jm800219f. - DOI - PubMed
- Ojima I.Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009.
- Salwiczek M.; Nyakatura E. K.; Gerling U. I. M.; Ye S.; Koksch B. Fluorinated amino acids: compatibility with native protein structures and effects on protein-protein interactions. Chem. Soc. Rev. 2012, 41, 2135–2171. 10.1039/c1cs15241f. - DOI - PubMed
-
- Foster R. W.; Lenz E. N.; Simpkins N. S.; Stead D. Organocatalytic Stereoconvergent Synthesis of α-CF3 Amides: Triketopiperazines and Their Heterocyclic Metamorphosis. Chem.—Eur. J. 2017, 23, 8810–8813. 10.1002/chem.201701548. - DOI - PubMed
- Saito A.; Kumagai N.; Shibasaki M. Cu/Pd Synergistic Dual Catalysis: Asymmetric α-Allylation of an α-CF3 Amide. Angew. Chem., Int. Ed. 2017, 56, 5551–5555. 10.1002/anie.201702113. - DOI - PubMed
- Yin L.; Brewitz L.; Kumagai N.; Shibasaki M. Catalytic Generation of α-CF3 Enolate: Direct Catalytic Asymmetric Mannich-Type Reaction of α-CF3 Amide. J. Am. Chem. Soc. 2014, 136, 17958–17961. 10.1021/ja511458k. - DOI - PubMed
- Matsuzawa A.; Noda H.; Kumagai N.; Shibasaki M. Direct Catalytic Asymmetric Aldol Addition of an α-CF3 Amide to Arylglyoxal Hydrates. J. Org. Chem. 2017, 82, 8304–8308. 10.1021/acs.joc.7b01381. - DOI - PubMed
- Brewitz L.; Arteaga F.; Yin L.; Alagiri K.; Kumagai N.; Shibasaki M. Direct Catalytic Asymmetric Mannich-Type Reaction of α- and β-Fluorinated Amides. J. Am. Chem. Soc. 2015, 137, 15929–15939. 10.1021/jacs.5b11064. - DOI - PubMed
LinkOut - more resources
Full Text Sources