Highly Efficient Recovery and Recycling of Cobalt from Spent Lithium-Ion Batteries Using an N-Methylurea-Acetamide Nonionic Deep Eutectic Solvent
- PMID: 36844576
- PMCID: PMC9948188
- DOI: 10.1021/acsomega.2c07780
Highly Efficient Recovery and Recycling of Cobalt from Spent Lithium-Ion Batteries Using an N-Methylurea-Acetamide Nonionic Deep Eutectic Solvent
Abstract
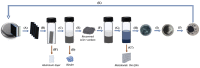
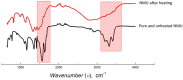
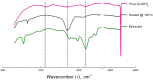
The growing demand for lithium-ion batteries (LiBs) for the electronic and automobile industries combined with the limited availability of key metal components, in particular cobalt, drives the need for efficient methods for the recovery and recycling of these materials from battery waste. Herein, we introduce a novel and efficient approach for the extraction of cobalt, and other metal components, from spent LiBs using a nonionic deep eutectic solvent (ni-DES) comprised of N-methylurea and acetamide under relatively mild conditions. Cobalt could be recovered from lithium cobalt oxide-based LiBs with an extraction efficiency of >97% and used to fabricate new batteries. The N-methylurea was found to act as both a solvent component and a reagent, the mechanism of which was elucidated.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Baum Z. J.; Bird R. E.; Yu X.; Ma J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Letters 2022, 7, 712–719. 10.1021/acsenergylett.1c02602. - DOI
- Armand M.; Axmann P.; Bresser D.; Copley M.; Edström K.; Ekberg C.; Guyomard D.; Lestriez B.; Novák P.; Petranikova M.; et al. Lithium-ion batteries – Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708 10.1016/j.jpowsour.2020.228708. - DOI
-
- McKerracher C.; O’Donovan A.; Soulopoulos N.; Grant A.; Mi S.; Doherty D.; Fisher R.; Cantor C.; Lyu J.; Ampofo K.. et al. Electric Vehicle Outlook 2022; BloombergNEF, 2022. https://about.bnef.com/electric-vehicle-outlook/ (accessed 2022 02-10-2022).
-
- Stone M.As electric vehicles take off, we’ll need to recycle their batteries. National Geographic, May 28, 2021. https://www.nationalgeographic.com/environment/article/electric-vehicles... (accessed 02-10-2022).
-
- Peters J. F.; Baumann M.; Zimmermann B.; Braun J.; Weil M. The environmental impact of Li-Ion batteries and the role of key parameters – A review. Renewable and Sustainable Energy Rev. 2017, 67, 491–506. 10.1016/j.rser.2016.08.039. - DOI
LinkOut - more resources
Full Text Sources

