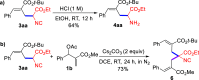
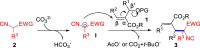Stereo- and Regiospecific SN2' Reaction of MBH Adducts with Isocyanoacetates: en Route to Transition-Metal-Free α-Allylation of Isocyanoacetates
- PMID: 36844594
- PMCID: PMC9948183
- DOI: 10.1021/acsomega.2c07581
Stereo- and Regiospecific SN2' Reaction of MBH Adducts with Isocyanoacetates: en Route to Transition-Metal-Free α-Allylation of Isocyanoacetates
Abstract
Herein, we report that under mild and transition-metal-free conditions an unprecedented and practical SN2' reaction of Morita-Baylis-Hillman adducts with isocyanoacetates takes place in a stereo- and regiospecific manner. This reaction which tolerates a wide variety of functionalities delivers transformable α-allylated isocyanoacetates in high efficiencies. Preliminary studies on the asymmetric version of this reaction indicate that ZnEt2/chiral amino alcohol combinations are an asymmetric catalytic system for this transformation, giving an enantioenriched α-allylated isocyanoacetate with a chiral quaternary carbon in a high yield.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Giustiniano M.; Basso A.; Mercalli V.; Massarotti A.; Novellino E.; Tron G. C.; Zhu J. P. To Each His Own: Isonitriles for All Flavors. Functionalized Isocyanides as Valuable Tools in Organic Synthesis. Chem. Soc. Rev. 2017, 46, 1295–1357. 10.1039/c6cs00444j. - DOI - PubMed
- Gulevich A. V.; Zhdanko A. G.; Orru R. V. A.; Nenajdenko V. G. Isocyanoacetate Derivatives: Synthesis, Reactivity, and Application. Chem. Rev. 2010, 110, 5235–5331. 10.1021/cr900411f. - DOI - PubMed
- Lygin A. V.; de Meijere A. Isocyanides in the Synthesis of Nitrogen Heterocycles. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. 10.1002/anie.201000723. - DOI - PubMed
- Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. - DOI - PubMed
- Zhu J. P. Recent Developments in the Isonitrile-Based Multicomponent Synthesis of Heterocycles. Eur. J. Org. Chem. 2003, 2003, 1133–1144. 10.1002/ejoc.200390167. - DOI
- Dömling A.; Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. - DOI - PubMed
-
- Yadav J. S.; Reddy P. A. N.; Reddy Y.; Meraj J. S.; Prasad A. R. Stereoselective Total Synthesis of Attenols A and B. Eur. J. Org. Chem. 2013, 2013, 6317–6324. 10.1002/ejoc.201300623. - DOI
- Brown A. L.; Churches Q. I.; Hutton C. A. Total Synthesis of Ustiloxin D Utilizing an Ammonia–Ugi Reaction. J. Org. Chem. 2015, 80, 9831–9837. 10.1021/acs.joc.5b01519. - DOI - PubMed
- Johnson R. E.; de Rond T.; Lindsay V. N. G.; Keasling J. D.; Sarpong R. Synthesis of Cycloprodigiosin Identifies the Natural Isolate as A Scalemic Mixture. Org. Lett. 2015, 17, 3474–3477. 10.1021/acs.orglett.5b01527. - DOI - PMC - PubMed
- Blair L. M.; Sperry J. Total Syntheses of (±)-Spiroindimicins B and C Enabled by A Late-Stage Schöllkopf–Magnus–Barton–Zard (SMBZ) Reaction. Chem. Commun. 2016, 52, 800–802. 10.1039/c5cc09060a. - DOI - PubMed
- Wei T.; Dixon D. J. Catalytic Stereoselective Total Synthesis of A Spiro-oxindole Alkaloid and the Pentacyclic Core of Tryptoquivalines. Chem. Commun. 2018, 54, 12860–12862. 10.1039/c8cc07479h. - DOI - PubMed
-
- Oe K.; Ohfune Y.; Shinada T. Short Total Synthesis of (−)-Kainic Acid. Org. Lett. 2014, 16, 2550–2553. 10.1021/ol5009526. - DOI - PubMed
- Liang X. F.; Gopalaswamy R.; Navas F.; Toone E. J.; Zhou P. A Scalable Synthesis of the Difluoromethyl-allo-threonyl Hydroxamate-Based LpxC Inhibitor LPC-058. J. Org. Chem. 2016, 81, 4393–4398. 10.1021/acs.joc.6b00589. - DOI - PMC - PubMed
-
- Riedel D.; Wurm T.; Graf K.; Rudolph M.; Rominger F.; Hashmi A. S. K. From Isonitriles to Unsaturated NHC Complexes of Gold, Palladium and Platinum. Adv. Synth. Catal. 2015, 357, 1515–1523. 10.1002/adsc.201401131. - DOI
- Knorn M.; Lutsker E.; Reiser O. Synthesis of New Chiral Bidentate Isonitrile–Acyclic Diaminocarbene Palladium(II) Compounds and Their Catalytic Activity. Organomet 2015, 34, 4515–4520. 10.1021/acs.organomet.5b00516. - DOI
-
- Peng X. J.; Ho Y. A.; Wang Z. P.; Shao P. L.; Zhao Y.; He Y. Formal [3+2] Cycloaddition of α-Unsubstituted Isocyanoacetates and Methyleneindolinones: Enantioselective Synthesis of Spirooxindoles. Org. Chem. Front. 2017, 4, 81–85. 10.1039/c6qo00555a. - DOI
- Zhang X.; Feng C. J.; Jiang T.; Li Y. F.; Pan L.; Xu X. X. Expedient and Divergent Tandem One-Pot Synthesis of Benz[e]indole and Spiro[indene-1,3’-pyrrole] Derivatives from Alkyne-Tethered Chalcones/Cinnamates and TosMIC. Org. Lett. 2015, 17, 3576–3579. 10.1021/acs.orglett.5b01676. - DOI - PubMed
- Zhao M. X.; Wei D. K.; Ji F. H.; Zhao X. L.; Shi M. Asymmetric Formal [3+2] Cycloaddition Reaction of α-Aryl Isocyanoesters with N-Aryl Maleimides by Bifunctional Cinchona Alkaloids-Based Squaramide/AgSbF6 Cooperative Catalysis. Chem. Asian J. 2012, 7, 2777–2781. 10.1002/asia.201200686. - DOI - PubMed
- Guo C.; Xue M. X.; Zhu M. K.; Gong L. Z. Organocatalytic Asymmetric Formal [3+2] Cycloaddition Reaction of Isocyanoesters to Nitroolefins Leading to Highly Optically Active Dihydropyrroles. Angew. Chem., Int. Ed. 2008, 47, 3414–3417. 10.1002/anie.200800003. - DOI - PubMed
- Buyck T.; Wang Q.; Zhu J. P. Catalytic Enantioselective Michael Addition of α-Aryl-α-Isocyanoacetates to Vinyl Selenone: Synthesis of α,α-Disubstituted α-Amino Acids and (+)- and (−)-Trigonoliimine A. Angew. Chem., Int. Ed. 2013, 52, 12714–12718. 10.1002/anie.201306663. - DOI - PubMed
- Zhao M. X.; Zhu H. K.; Dai T. L.; Shi M. Cinchona Alkaloid Squaramide-Catalyzed Asymmetric Michael Addition of α-Aryl Isocyanoacetates to β-Trifluoromethylated Enones and Its Applications in the Synthesis of Chiral β-Trifluoromethylated Pyrrolines. J. Org. Chem. 2015, 80, 11330–11338. 10.1021/acs.joc.5b01829. - DOI - PubMed
- Wang Z. P.; Li Z. R.; Wu Q.; Peng X. J.; Shao P. L.; He Y. Enantioselective Synthesis of Alkylthioetherpyrrolidine Derivatives via [3+2] Cycloaddition of α-Thioacrylates with Isocyanoacetates. J. Org. Chem. 2017, 82, 12869–12876. 10.1021/acs.joc.7b02266. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials