Acylpyrazoline-Based Third-Generation Selective Antichlamydial Compounds with Enhanced Potency
- PMID: 36844602
- PMCID: PMC9947980
- DOI: 10.1021/acsomega.2c06992
Acylpyrazoline-Based Third-Generation Selective Antichlamydial Compounds with Enhanced Potency
Abstract
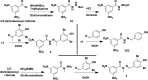
Chlamydiae are obligate intracellular Gram-negative bacteria and widespread pathogens in humans and animals. Broad-spectrum antibiotics are currently used to treat chlamydial infections. However, broad-spectrum drugs also kill beneficial bacteria. Recently, two generations of benzal acylhydrazones have been shown to selectively inhibit chlamydiae without toxicity to human cells and lactobacilli, which are dominating, beneficial bacteria in the vagina of reproductive-age women. Here, we report the identification of two acylpyrazoline-based third-generation selective antichlamydials (SACs). With minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) of 10-25 μM against Chlamydia trachomatis and Chlamydia muridarum, these new antichlamydials are 2- to 5-fold more potent over the benzal acylhydrazone-based second-generation selective antichlamydial lead SF3. Both acylpyrazoline-based SACs are well tolerated by Lactobacillus, Escherichia coli, Klebsiella, and Salmonella as well as host cells. These third-generation selective antichlamydials merit further evaluation for therapeutic application.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
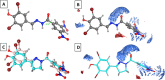

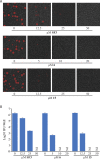

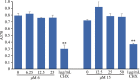
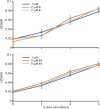

References
-
- CDC, CDC fact sheet. STD trends in the United States: 2011 national data for chlamydia, gonorrhea, and syphilis. 2013.
Grants and funding
LinkOut - more resources
Full Text Sources

