Cirrhosis is associated with lower serological responses to COVID-19 vaccines in patients with chronic liver disease
- PMID: 36844943
- PMCID: PMC9939238
- DOI: 10.1016/j.jhepr.2023.100697
Cirrhosis is associated with lower serological responses to COVID-19 vaccines in patients with chronic liver disease
Erratum in
-
Erratum Regarding Previously Published Articles.JHEP Rep. 2024 May 18;6(6):101097. doi: 10.1016/j.jhepr.2024.101097. eCollection 2024 Jun. JHEP Rep. 2024. PMID: 38978774 Free PMC article.
Abstract
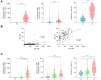
Background & aims: The response of patients with chronic liver disease (CLD) to COVID-19 vaccines remains unclear. Our aim was to assess the humoral immune response and efficacy of two-dose COVID-19 vaccines among patients with CLD of different aetiologies and disease stages.
Methods: A total of 357 patients were recruited in clinical centres from six European countries, and 132 healthy volunteers served as controls. Serum IgG (nM), IgM (nM), and neutralising antibodies (%) against the Wuhan-Hu-1, B.1.617, and B.1.1.529 SARS-CoV-2 spike proteins were determined before vaccination (T0) and 14 days (T2) and 6 months (T3) after the second-dose vaccination. Patients fulfilling inclusion criteria at T2 (n = 212) were stratified into 'low' or 'high' responders according to IgG levels. Infection rates and severity were collected throughout the study.
Results: Wuhan-Hu-1 IgG, IgM, and neutralisation levels significantly increased from T0 to T2 in patients vaccinated with BNT162b2 (70.3%), mRNA-1273 (18.9%), or ChAdOx1 (10.8%). In multivariate analysis, age, cirrhosis, and type of vaccine (ChAdOx1 > BNT162b2 > mRNA-1273) predicted 'low' humoral response, whereas viral hepatitis and antiviral therapy predicted 'high' humoral response. Compared with Wuhan-Hu-1, B.1.617 and, further, B.1.1.529 IgG levels were significantly lower at both T2 and T3. Compared with healthy individuals, patients with CLD presented with lower B.1.1.529 IgGs at T2 with no additional key differences. No major clinical or immune IgG parameters associated with SARS-CoV-2 infection rates or vaccine efficacy.
Conclusions: Patients with CLD and cirrhosis exhibit lower immune responses to COVID-19 vaccination, irrespective of disease aetiology. The type of vaccine leads to different antibody responses that appear not to associate with distinct efficacy, although this needs validation in larger cohorts with a more balanced representation of all vaccines.
Impact and implications: In patients with CLD vaccinated with two-dose vaccines, age, cirrhosis, and type of vaccine (Vaxzevria > Pfizer BioNTech > Moderna) predict a 'lower' humoral response, whereas viral hepatitis aetiology and prior antiviral therapy predict a 'higher' humoral response. This differential response appears not to associate with SARS-CoV-2 infection incidence or vaccine efficacy. However, compared with Wuhan-Hu-1, humoral immunity was lower for the Delta and Omicron variants, and all decreased after 6 months. As such, patients with CLD, particularly those older and with cirrhosis, should be prioritised for receiving booster doses and/or recently approved adapted vaccines.
Keywords: COVID-19 vaccine; Chronic liver disease; Cirrhosis; Humoral immunity; SARS-CoV-2.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Albillos A., Martin-Mateos R., Van der Merwe S., Wiest R., Jalan R., Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112–134. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

