Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: a retrospective cohort study
- PMID: 36844970
- PMCID: PMC9940330
- DOI: 10.1016/S2665-9913(23)00006-1
Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: a retrospective cohort study
Abstract
Background: Some patients with systemic autoimmune rheumatic disease and immunosuppression might still be at risk of severe COVID-19. The effect of outpatient SARS-CoV-2 treatments on COVID-19 outcomes among patients with systemic autoimmune rheumatic disease is unclear. We aimed to evaluate temporal trends, severe outcomes, and COVID-19 rebound among patients with systemic autoimmune rheumatic disease and COVID-19 who received outpatient SARS-CoV-2 treatment compared with those who did not receive outpatient treatment.
Methods: We did a retrospective cohort study at Mass General Brigham Integrated Health Care System, Boston, MA, USA. We included patients aged 18 years or older with a pre-existing systemic autoimmune rheumatic disease, who had COVID-19 onset between Jan 23 and May 30, 2022. We identified COVID-19 by positive PCR or antigen test (index date defined as the date of first positive test) and systemic autoimmune rheumatic diseases using diagnosis codes and immunomodulator prescription. Outpatient SARS-CoV-2 treatments were confirmed by medical record review. The primary outcome was severe COVID-19, defined as hospitalisation or death within 30 days after the index date. COVID-19 rebound was defined as documentation of a negative SARS-CoV-2 test after treatment followed by a newly positive test. The association of outpatient SARS-CoV-2 treatment versus no outpatient treatment with severe COVID-19 outcomes was assessed using multivariable logistic regression.
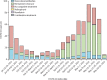
Findings: Between Jan 23 and May 30, 2022, 704 patients were identified and included in our analysis (mean age 58·4 years [SD 15·9]; 536 [76%] were female and 168 [24%] were male, 590 [84%] were White and 39 [6%] were Black, and 347 [49%] had rheumatoid arthritis). Outpatient SARS-CoV-2 treatments increased in frequency over calendar time (p<0·0001). A total of 426 (61%) of 704 patients received outpatient treatment (307 [44%] with nirmatrelvir-ritonavir, 105 [15%] with monoclonal antibodies, five [1%] with molnupiravir, three [<1%] with remdesivir, and six [1%] with combination treatment). There were nine (2·1%) hospitalisations or deaths among 426 patients who received outpatient treatment compared with 49 (17·6%) among 278 who did not receive outpatient treatment (odds ratio [adjusted for age, sex, race, comorbidities, and kidney function] 0·12, 95% CI 0·05-0·25). 25 (7·9%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
Interpretation: Outpatient treatment was associated with lower odds of severe COVID-19 outcomes compared with no outpatient treatment. These findings highlight the importance of outpatient SARS-CoV-2 treatment for patients with systemic autoimmune rheumatic disease and COVID-19 and the need for further research on COVID-19 rebound.
Funding: None.
© 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
NJP reports consulting fees from FVC Health. MEW reports research support from Bristol Myers Squibb, Sanofi, Eli Lilly, Amgen; consulting fees from Abbvie, Aclaris, Amgen, Bristol Myers Squibb, Corevitas, EQRx, Genosco, GlaxoSmithKline, Gilead, Horizon, Janssen, Johnson & Johnson, Eli Lilly, Pfizer, Roche, Sanofi, Scipher, Set Point, and Tremeau; and stock options in Can-Fite, Inmediz, and Scipher, unrelated to this work. ZSW reports research support from Bristol Myers Squibb and Principia Sanofi; consulting fees from Zenas Biopharma, Visterra Otsuka, Horizon, Sanofi, Shionogi, Viela Bio, and MedPace; and has participated on advisory boards for Sanofi and Horizon. JAS reports research support from Bristol Myers Squibb; and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. All other authors declare no competing interests.
Figures

Update of
-
Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: A retrospective cohort study.medRxiv [Preprint]. 2022 Oct 30:2022.10.27.22281629. doi: 10.1101/2022.10.27.22281629. medRxiv. 2022. Update in: Lancet Rheumatol. 2023 Mar;5(3):e139-e150. doi: 10.1016/S2665-9913(23)00006-1. PMID: 36324801 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

