Integrative network pharmacology and in silico analyses identify the anti-omicron SARS-CoV-2 potential of eugenol
- PMID: 36845041
- PMCID: PMC9937729
- DOI: 10.1016/j.heliyon.2023.e13853
Integrative network pharmacology and in silico analyses identify the anti-omicron SARS-CoV-2 potential of eugenol
Abstract
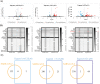
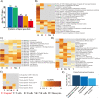
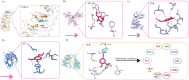
Eugenol as a natural product is the source of isoniazid, and purified eugenol is extensively used in the cosmetics industry and the productive processes of edible spices. Accumulating evidence suggested that eugenol exerted potent anti-microorganism and anti-inflammation effects. Application of eugenol effectively reduced the risk of atherosclerosis, arterial embolism, and Type 2 diabetes. A previous study confirmed that treatment with eugenol attenuated lung inflammation and improved heart functions in SARS-CoV-2 spike S1-intoxicated mice. In addition to the study, based on a series of public datasets, computational analyses were conducted to characterize the acting targets of eugenol and the functional roles of these targets in COVID-19. The binding capacities of eugenol to conservative sites of SARS-CoV-2 like RNA-dependent RNA polymerase (RdRp) and mutable site as spike (S) protein, were calculated by using molecular docking following the molecular dynamics simulation with RMSD, RMSF, and MM-GBSA methods. The results of network pharmacology indicated that six targets, including PLAT, HMOX1, NUP88, CTSL, ITGB1 andTMPRSS2 were eugenol-SARS-CoV-2 interacting proteins. The omics results of in-silico study further implicated that eugenol increased the expression of SCARB1, HMOX1 and GDF15, especially HMOX1, which were confirmed the potential interacting targets between eugenol and SARS-CoV-2 antigens. Enrichment analyses indicated that eugenol exerted extensive biological effects such as regulating immune infiltration of macrophage, lipid localization, monooxyenase activity, iron ion binding and PPAR signaling. The results of the integrated analysis of eugenol targets and immunotranscription profile of COVID-19 cases shows that eugenol also plays an important role in strengthen of immunologic functions and regulating cytokine signaling. As a complement to the integrated analysis, the results of molecular docking indicated the potential binding interactions between eugenol and four proteins relating to cytokine production/release and the function of T type lymphocytes, including human TLR-4, TCR, NF-κB, JNK and AP-1. Furthermore, results of molecular docking and molecular dynamics (100ns) simulations implicated that stimulated modification of eugenol to the SARS-CoV-2 Omicron Spike-ACE2 complex, especially for human ACE2, and the molecular interaction of eugenol to SARS-CoV-2 RdRp, were no less favorable than two positive controls, molnupiravir and nilotinib. Dynamics (200ns) simulations indicated that the binding capacities and stabilities of eugenol to finger subdomain of RdRp is no less than molnupiravir. However, the simulated binding capacity of eugenol to SARS-CoV-2 wild type RBD and Omicron mutant RBD were less than nilotinib. Eugenol was predicted to have more favor LD50 value and lower cytotoxicity than two positive controls, and eugenol can pass through the blood-brain barrier (BBB). In a brief, eugenol is helpful for attenuating systemic inflammation induced by SARS-CoV-2 infection, due to the direct interaction of eugenol to SARS-CoV-2 proteins and extensive bio-manipulation of pro-inflammatory factors. This study carefully suggests eugenol is a candidate compound of developing drugs and supplement agents against SARS-CoV-2 and its Omicron variants.
Keywords: COVID-19; Eugenol; Omicron; RNA-Dependent RNA polymerase; SARS-CoV-2; Spike protein.
© 2023 The Author.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




















References
-
- Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., Reis B.Y., Balicer R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. - DOI - PMC - PubMed
-
- Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., Louca P., May A., Figueiredo J.C., Hu C., Molteni E., Canas L., Österdahl M.F., Modat M., Sudre C.H., Fox B., Hammers A., Wolf J., Capdevila J., Chan A.T., David S.P., Steves C.J., Ourselin S., Spector T.D. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. - DOI - PMC - PubMed
-
- Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., Murray B., Kerfoot E., Chen L., Deng J., Österdahl M.F., Cheetham N.J., Drew D.A., Nguyen L.H., Pujol J.C., Hu C., Selvachandran S., Polidori L., May A., Wolf J., Chan A.T., Hammers A., Duncan E.L., Spector T.D., Ourselin S., Steves C.J. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022;22(1):43–55. doi: 10.1016/S1473-3099(21)00460-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

