Impact of dielectric barrier discharge cold plasma on the lipid oxidation, color stability, and protein structures of myoglobin-added washed pork muscle
- PMID: 36845053
- PMCID: PMC9947400
- DOI: 10.3389/fnut.2023.1137457
Impact of dielectric barrier discharge cold plasma on the lipid oxidation, color stability, and protein structures of myoglobin-added washed pork muscle
Abstract
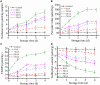
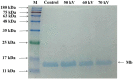
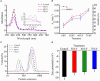
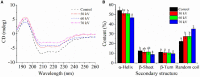
Cold plasma has been considered a novel non-thermal processing technique and attracted a high attention by the food industry. In this study, the influences of dielectric barrier discharge cold plasma (DBD-CP) on the myoglobin (Mb)-added washed pork muscle (WPM) were evaluated. The electrophoresis pattern, autoxidation, and secondary structure of Mb were analyzed. The results found that DBD-CP caused the decrease of the redness and total sulfhydryl (T-SH) in WPM, while the increase of non-heme, peroxide value (PV), and thiobarbituric acid reactive substances (TBARS), suggested that treatment triggered protein oxidation and heme degradation. Additionally, DBD-CP treatment enhanced the autoxidation of Mb, induced the release of intact heme from the globin, rearranged the charged groups, and promoted Mb aggregation. The transformation of α-helix into the random coil of Mb demonstrated that DBD-CP weakened the tensile strength. Overall, data indicated that DBD-CP promoted autoxidation and changed the secondary structure of Mb, accelerating Mb-mediated lipid oxidation in WPM. Thus, further studies about the optimization of processing conditions by DBD-CP need to be performed.
Keywords: bioactive compounds; dielectric barrier discharge cold plasma; lipid oxidation; myoglobin; novel non-thermal processing technology; secondary structure.
Copyright © 2023 Wang, Wang, Wang, Yan, Zhuang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Changes in color, myoglobin, and lipid oxidation in beef patties treated by dielectric barrier discharge cold plasma during storage.Meat Sci. 2021 Jun;176:108456. doi: 10.1016/j.meatsci.2021.108456. Epub 2021 Feb 13. Meat Sci. 2021. PMID: 33621829
-
Mechanisms of heme protein-mediated lipid oxidation using hemoglobin and myoglobin variants in raw and heated washed muscle.J Agric Food Chem. 2006 Oct 18;54(21):8271-80. doi: 10.1021/jf061231d. J Agric Food Chem. 2006. PMID: 17032039
-
Effect of in-package high voltage dielectric barrier discharge on microbiological, color and oxidation properties of pork in modified atmosphere packaging during storage.Meat Sci. 2019 Mar;149:107-113. doi: 10.1016/j.meatsci.2018.11.016. Epub 2018 Nov 22. Meat Sci. 2019. PMID: 30502608
-
Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products.Compr Rev Food Sci Food Saf. 2021 May;20(3):2626-2659. doi: 10.1111/1541-4337.12740. Epub 2021 Apr 20. Compr Rev Food Sci Food Saf. 2021. PMID: 33876887 Review.
-
Dielectric Barrier Discharge for Solid Food Applications.Nutrients. 2022 Nov 3;14(21):4653. doi: 10.3390/nu14214653. Nutrients. 2022. PMID: 36364914 Free PMC article. Review.
Cited by
-
Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine.Int J Mol Sci. 2023 Nov 2;24(21):15889. doi: 10.3390/ijms242115889. Int J Mol Sci. 2023. PMID: 37958872 Free PMC article. Review.
-
Effects of Supercritical CO2 Treatment on Color, Lipid Oxidation, Heme Iron, Non-Heme Iron and Metmyoglobin Contents in Ground Pork.Food Sci Anim Resour. 2024 Mar;44(2):408-429. doi: 10.5851/kosfa.2024.e77. Epub 2024 Mar 1. Food Sci Anim Resour. 2024. PMID: 38764518 Free PMC article.
-
Salvia officinalis L. and Salvia sclarea Essential Oils: Chemical Composition, Biological Activities and Preservative Effects against Listeria monocytogenes Inoculated into Minced Beef Meat.Plants (Basel). 2023 Sep 25;12(19):3385. doi: 10.3390/plants12193385. Plants (Basel). 2023. PMID: 37836125 Free PMC article.
-
Lipid oxidation in foods and its implications on proteins.Front Nutr. 2023 Jun 15;10:1192199. doi: 10.3389/fnut.2023.1192199. eCollection 2023. Front Nutr. 2023. PMID: 37396138 Free PMC article. Review.
-
Aflatoxin B1, ochratoxin A, and fumonisin B1 detoxification from poultry feeds by corona discharge application.J Adv Vet Anim Res. 2024 Dec 26;11(4):819-834. doi: 10.5455/javar.2024.k834. eCollection 2024 Dec. J Adv Vet Anim Res. 2024. PMID: 40013271 Free PMC article.
References
-
- Misra NN, Pankaj SK, Segat A, Ishikawa K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci Technol. (2016) 55:39–47. 10.1016/j.tifs.2016.07.001 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

