Wild-type SARS-CoV-2 neutralizing immunity decreases across variants and over time but correlates well with diagnostic testing
- PMID: 36845123
- PMCID: PMC9945103
- DOI: 10.3389/fimmu.2023.1055429
Wild-type SARS-CoV-2 neutralizing immunity decreases across variants and over time but correlates well with diagnostic testing
Abstract
Importance: The degree of immune protection against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants provided by infection versus vaccination with wild-type virus remains unresolved, which could influence future vaccine strategies. The gold-standard for assessing immune protection is viral neutralization; however, few studies involve a large-scale analysis of viral neutralization against the Omicron variant by sera from individuals infected with wild-type virus.
Objectives: 1) To define the degree to which infection versus vaccination with wild-type SARS-CoV-2 induced neutralizing antibodies against Delta and Omicron variants.2) To determine whether clinically available data, such as infection/vaccination timing or antibody status, can predict variant neutralization.
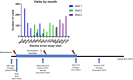
Methods: We examined a longitudinal cohort of 653 subjects with sera collected three times at 3-to-6-month intervals from April 2020 to June 2021. Individuals were categorized according to SARS-CoV-2 infection and vaccination status. Spike and nucleocapsid antibodies were detected via ADVIA Centaur® (Siemens) and Elecsys® (Roche) assays, respectively. The Healgen Scientific® lateral flow assay was used to detect IgG and IgM spike antibody responses. Pseudoviral neutralization assays were performed on all samples using human ACE2 receptor-expressing HEK-293T cells infected with SARS-CoV-2 spike protein pseudotyped lentiviral particles for wild-type (WT), B.1.617.2 (Delta), and B.1.1.529 (Omicron) variants.
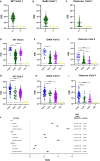
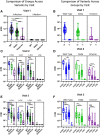
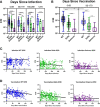
Results: Vaccination after infection led to the highest neutralization titers at all timepoints for all variants. Neutralization was also more durable in the setting of prior infection versus vaccination alone. Spike antibody clinical testing effectively predicted neutralization for wild-type and Delta. However, nucleocapsid antibody presence was the best independent predictor of Omicron neutralization. Neutralization of Omicron was lower than neutralization of either wild-type or Delta virus across all groups and timepoints, with significant activity only present in patients that were first infected and later immunized.
Conclusions: Participants having both infection and vaccination with wild-type virus had the highest neutralizing antibody levels against all variants and had persistence of activity. Neutralization of WT and Delta virus correlated with spike antibody levels against wild-type and Delta variants, but Omicron neutralization was better correlated with evidence of prior infection. These data help explain why 'breakthrough' Omicron infections occurred in previously vaccinated individuals and suggest better protection is observed in those with both vaccination and previous infection. This study also supports the concept of future SARS-CoV-2 Omicron-specific vaccine boosters.
Keywords: COVID-19; SARS-CoV-2; antibody; nucleocapsid; spike; vaccine; variant of concern; viral neutralization.
Copyright © 2023 O’Shea, Schuler, Chen, Troost, Wong, Chen, O’Shea, Peng, Gherasim, Manthei, Valdez, Baldwin and Baker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19).2023 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 34033342 Free Books & Documents.
-
Vaccination and Omicron BA.1/BA.2 Convalescence Enhance Systemic but Not Mucosal Immunity against BA.4/5.Microbiol Spectr. 2023 Jun 15;11(3):e0516322. doi: 10.1128/spectrum.05163-22. Epub 2023 Apr 26. Microbiol Spectr. 2023. PMID: 37098903 Free PMC article.
-
Pre-Omicron Vaccine Breakthrough Infection Induces Superior Cross-Neutralization against SARS-CoV-2 Omicron BA.1 Compared to Infection Alone.Int J Mol Sci. 2022 Jul 12;23(14):7675. doi: 10.3390/ijms23147675. Int J Mol Sci. 2022. PMID: 35887023 Free PMC article.
-
Infection-mediated immune response in SARS-CoV-2 breakthrough infection and implications for next-generation COVID-19 vaccine development.Vaccine. 2024 Feb 27;42(6):1401-1406. doi: 10.1016/j.vaccine.2024.01.088. Epub 2024 Feb 2. Vaccine. 2024. PMID: 38310015 Review.
-
The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern.Front Med (Lausanne). 2022 May 20;9:849217. doi: 10.3389/fmed.2022.849217. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35669924 Free PMC article. Review.
Cited by
-
Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays.Vaccines (Basel). 2023 Nov 7;11(11):1694. doi: 10.3390/vaccines11111694. Vaccines (Basel). 2023. PMID: 38006026 Free PMC article.
-
Longitudinal analysis of humoral and cellular immunity in SARS-CoV-2 exposed families.Sci Rep. 2025 Jul 18;15(1):26041. doi: 10.1038/s41598-025-07739-3. Sci Rep. 2025. PMID: 40681584 Free PMC article.
-
Timing of maternal vaccination against COVID-19 for effective protection of neonates: cohort study.Front Immunol. 2024 Jul 8;15:1359209. doi: 10.3389/fimmu.2024.1359209. eCollection 2024. Front Immunol. 2024. PMID: 39040104 Free PMC article.
-
Changes in peripheral blood mononuclear cell electrical properties in response to viral exposure and vaccination.Sci Rep. 2025 Jul 9;15(1):24583. doi: 10.1038/s41598-025-08724-6. Sci Rep. 2025. PMID: 40628860 Free PMC article.
-
Antibody longevity and waning following COVID-19 vaccination in a 1-year longitudinal cohort in Bangladesh.Sci Rep. 2024 May 20;14(1):11467. doi: 10.1038/s41598-024-61922-6. Sci Rep. 2024. PMID: 38769324 Free PMC article.
References
-
- WHO . WHO coronavirus (COVID-19) dashboard (2021). Available at: https://covid19.who.int/table.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

