Chrysanthemum sporopollenin: A novel vaccine delivery system for nasal mucosal immunity
- PMID: 36845130
- PMCID: PMC9947463
- DOI: 10.3389/fimmu.2023.1132129
Chrysanthemum sporopollenin: A novel vaccine delivery system for nasal mucosal immunity
Abstract
Objective: Mucosal immunization was an effective defender against pathogens. Nasal vaccines could activate both systemic and mucosal immunity to trigger protective immune responses. However, due to the weak immunogenicity of nasal vaccines and the lack of appropriate antigen carriers, very few nasal vaccines have been clinically approved for human use, which was a major barrier to the development of nasal vaccines. Plant-derived adjuvants are promising candidates for vaccine delivery systems due to their relatively safe immunogenic properties. In particular, the distinctive structure of pollen was beneficial to the stability and retention of antigen in the nasal mucosa.
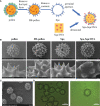
Methods: Herein, a novel wild-type chrysanthemum sporopollenin vaccine delivery system loaded with a w/o/w emulsion containing squalane and protein antigen was fabricated. The unique internal cavities and the rigid external walls within the sporopollenin skeleton construction could preserve and stabilize the inner proteins. The external morphological characteristics were suitable for nasal mucosal administration with high adhesion and retention.
Results: Secretory IgA antibodies in the nasal mucosa can be induced by the w/o/w emulsion with the chrysanthemum sporopollenin vaccine delivery system. Moreover, the nasal adjuvants produce a stronger humoral response (IgA and IgG) compared to squalene emulsion adjuvant. Mucosal adjuvant benefited primarily from prolongation of antigens in the nasal cavity, improvement of antigen penetration in the submucosa and promotion of CD8+ T cells in spleen.
Disccusion: Based on effective delivering both the adjuvant and the antigen, the increase of protein antigen stability and the realization of mucosal retention, the chrysanthemum sporopollenin vaccine delivery system has the potential to be a promising adjuvant platform. This work provide a novel idea for the fabrication of protein-mucosal delivery vaccine.
Keywords: adhesion; chrysanthemum; nasal mucosal immunity; sporopollenin; vaccine delivery system.
Copyright © 2023 Liu, Yan, Li, Du, Zhu, Ye, Cao, Dong, Li, Xu, Bai, Li, Zhang, Wang, Li, Sun and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer YX declared a shared affiliation, with with several of the authors, JL, ZYC, JWC, XZY, to the handling editor at the time of the review.
Figures

Similar articles
-
Oil-in-ionic liquid nanoemulsion-based intranasal delivery system for influenza split-virus vaccine.J Control Release. 2022 Jun;346:380-391. doi: 10.1016/j.jconrel.2022.04.036. Epub 2022 Apr 30. J Control Release. 2022. PMID: 35483639
-
Fabrication of chitosan-based emulsion as an adjuvant to enhance nasal mucosal immune responses.Int J Biol Macromol. 2024 Jun;272(Pt 2):132913. doi: 10.1016/j.ijbiomac.2024.132913. Epub 2024 Jun 6. Int J Biol Macromol. 2024. PMID: 38851606
-
Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity.Eur J Pharm Biopharm. 2015 Jun;93:149-64. doi: 10.1016/j.ejpb.2015.03.024. Epub 2015 Mar 26. Eur J Pharm Biopharm. 2015. PMID: 25818119
-
Nasal and pulmonary vaccine delivery using particulate carriers.Expert Opin Drug Deliv. 2015 Jun;12(6):993-1008. doi: 10.1517/17425247.2015.1044435. Epub 2015 May 8. Expert Opin Drug Deliv. 2015. PMID: 25952104 Review.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
Cited by
-
Intranasally administered whole virion inactivated vaccine against clade 2.3.4.4b H5N1 influenza virus with optimized antigen and increased cross-protection.Virol J. 2025 May 5;22(1):131. doi: 10.1186/s12985-025-02760-4. Virol J. 2025. PMID: 40320528 Free PMC article.
-
Stimulus triggered release of actives from composite microcapsules based on sporopollenin from Lycopodium clavatum.RSC Adv. 2024 Mar 28;14(15):10280-10289. doi: 10.1039/d4ra00236a. eCollection 2024 Mar 26. RSC Adv. 2024. PMID: 38549792 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

