Comparison of antibody responses before and after booster doses with the Pfizer-BioNTech or Oxford-AstraZeneca vaccines in healthcare workers in Thailand
- PMID: 36845212
- PMCID: PMC9940472
- DOI: 10.1016/j.jvacx.2023.100277
Comparison of antibody responses before and after booster doses with the Pfizer-BioNTech or Oxford-AstraZeneca vaccines in healthcare workers in Thailand
Abstract
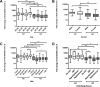
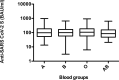
The severe acute respiratory syndrome 2 (SARS-CoV-2) has spread rapidly worldwide, not only causing significant morbidity and mortality but also dramatically increasing health care spending. To manage this in Thailand, healthcare workers first received two doses of the CoronaVac vaccine followed by a booster vaccine with either BNT162b2 vaccine (Pfizer-BioNTech; PZ) or ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca; AZ). Given that the difference in anti-SARS-CoV-2 levels following vaccination may vary depending on the vaccine and on demographic characteristics, we measured the antibody response after the second CoronaVac dose and after the booster with either the PZ or AZ vaccine. Our results in 473 healthcare workers show that the variation in antibody response to the full CoronaVac dose depends on demographic characteristics such as age, gender, body mass index, and underlying disease. After receiving a booster dose, anti-SARS-CoV-2 levels were significantly higher in participants who received the PZ vaccine than in people who received the AZ vaccine. Overall, however, receiving a booster dose of either the PZ or AZ vaccine promoted strong antibody responses, even in the old and those with obesity or diabetes mellitus. In conclusion, our results support the use of a booster vaccination program after full vaccination with the CoronaVac vaccine. This approach effectively enhances immunity against SARS-CoV-2, especially in clinically vulnerable groups and healthcare workers.
Keywords: Anti-SARS-CoV-2 S; COVID-19; Oxford–AstraZeneca; Pfizer-BioNTech; Vaccine.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- WHO. WHO coronavirus disease (COVID-19) dashboard. 2021. https://covid19whoint/ (accessed January, 10 2023).
LinkOut - more resources
Full Text Sources
Miscellaneous

