Functional imaging of the exposed brain
- PMID: 36845427
- PMCID: PMC9947297
- DOI: 10.3389/fnins.2023.1087912
Functional imaging of the exposed brain
Abstract
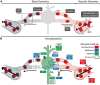
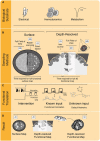
When the brain is exposed, such as after a craniotomy in neurosurgical procedures, we are provided with the unique opportunity for real-time imaging of brain functionality. Real-time functional maps of the exposed brain are vital to ensuring safe and effective navigation during these neurosurgical procedures. However, current neurosurgical practice has yet to fully harness this potential as it pre-dominantly relies on inherently limited techniques such as electrical stimulation to provide functional feedback to guide surgical decision-making. A wealth of especially experimental imaging techniques show unique potential to improve intra-operative decision-making and neurosurgical safety, and as an added bonus, improve our fundamental neuroscientific understanding of human brain function. In this review we compare and contrast close to twenty candidate imaging techniques based on their underlying biological substrate, technical characteristics and ability to meet clinical constraints such as compatibility with surgical workflow. Our review gives insight into the interplay between technical parameters such sampling method, data rate and a technique's real-time imaging potential in the operating room. By the end of the review, the reader will understand why new, real-time volumetric imaging techniques such as functional Ultrasound (fUS) and functional Photoacoustic Computed Tomography (fPACT) hold great clinical potential for procedures in especially highly eloquent areas, despite the higher data rates involved. Finally, we will highlight the neuroscientific perspective on the exposed brain. While different neurosurgical procedures ask for different functional maps to navigate surgical territories, neuroscience potentially benefits from all these maps. In the surgical context we can uniquely combine healthy volunteer studies, lesion studies and even reversible lesion studies in in the same individual. Ultimately, individual cases will build a greater understanding of human brain function in general, which in turn will improve neurosurgeons' future navigational efforts.
Keywords: brain mapping; craniotomy; exposed brain; functional imaging; functional ultrasound; imaging techniques; neuronal activity; surgical decision-making.
Copyright © 2023 Soloukey, Vincent, Smits, De Zeeuw, Koekkoek, Dirven and Kruizinga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Almasian M., Wilk L. S., Bloemen P. R., van Leeuwen T. G., ter Laan M., Aalders M. C. G. (2019). Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J. Biophoton. 12:e201900037. 10.1002/jbio.201900037 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

