Up-regulation of HCN2 channels in a thalamocortical circuit mediates allodynia in mice
- PMID: 36846300
- PMCID: PMC9945406
- DOI: 10.1093/nsr/nwac275
Up-regulation of HCN2 channels in a thalamocortical circuit mediates allodynia in mice
Abstract
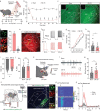
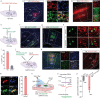
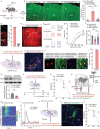
Chronic pain is a significant problem that afflicts individuals and society, and for which the current clinical treatment is inadequate. In addition, the neural circuit and molecular mechanisms subserving chronic pain remain largely uncharacterized. Herein we identified enhanced activity of a glutamatergic neuronal circuit that encompasses projections from the ventral posterolateral nucleus (VPLGlu) to the glutamatergic neurons of the hindlimb primary somatosensory cortex (S1HLGlu), driving allodynia in mouse models of chronic pain. Optogenetic inhibition of this VPLGlu→S1HLGlu circuit reversed allodynia, whereas the enhancement of its activity provoked hyperalgesia in control mice. In addition, we found that the expression and function of the HCN2 (hyperpolarization-activated cyclic nucleotide-gated channel 2) were increased in VPLGlu neurons under conditions of chronic pain. Using in vivo calcium imaging, we demonstrated that downregulation of HCN2 channels in the VPLGlu neurons abrogated the rise in S1HLGlu neuronal activity while alleviating allodynia in mice with chronic pain. With these data, we propose that dysfunction in HCN2 channels in the VPLGlu→S1HLGlu thalamocortical circuit and their upregulation occupy essential roles in the development of chronic pain.
Keywords: HCN2 channels; chronic pain; in vivo recordings; neural circuit.
© The Author(s) 2022. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–59. - PMC - PubMed
LinkOut - more resources
Full Text Sources
