The superior cervical ganglion is involved in chronic chemoreflex sensitization during recovery from acute lung injury
- PMID: 36846321
- PMCID: PMC9944401
- DOI: 10.3389/fphys.2023.1101408
The superior cervical ganglion is involved in chronic chemoreflex sensitization during recovery from acute lung injury
Abstract
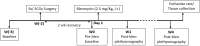
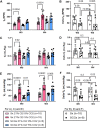
Introduction: Acute lung injury (ALI) initiates an inflammatory cascade that impairs gas exchange, induces hypoxemia, and causes an increase in respiratory rate (fR). This stimulates the carotid body (CB) chemoreflex, a fundamental protective reflex that maintains oxygen homeostasis. Our previous study indicated that the chemoreflex is sensitized during the recovery from ALI. The superior cervical ganglion (SCG) is known to innervate the CB, and its electrical stimulation has been shown to significantly sensitize the chemoreflex in hypertensive and normotensive rats. We hypothesized that the SCG is involved in the chemoreflex sensitization post-ALI. Methods: We performed a bilateral SCG ganglionectomy (SCGx) or sham-SCGx (Sx) in male Sprague Dawley rats 2 weeks before inducing ALI (Week -2 i.e., W-2). ALI was induced using a single intra-tracheal instillation of bleomycin (bleo) (day 1). Resting-fR, Vt (Tidal Volume), and V̇ E (Minute Ventilation) were measured. The chemoreflex response to hypoxia (10% O2, 0% CO2) and normoxic-hypercapnia (21% O2, 5% CO2) were measured before surgery on W (-3), before bleo administration on W0 and on W4 post-bleo using whole-body plethysmography (WBP). Results: SCGx did not affect resting fR, Vt and V̇E as well as the chemoreflex responses to hypoxia and normoxic hypercapnia in either group prior to bleo. There was no significant difference in ALI-induced increase in resting fR between Sx and SCGx rats at W1 post-bleo. At W4 post-bleo, there were no significant differences in resting fR, Vt, and V̇E between Sx and SCGx rats. Consistent with our previous study, we observed a sensitized chemoreflex (delta fR) in response to hypoxia and normoxic hypercapnia in Sx rats at W4 post-bleo. However, at the same time, compared to Sx rats, the chemoreflex sensitivity was significantly less in SCGx rats in response to either hypoxia or normoxic hypercapnia. Discussion: These data suggest that SCG is involved in the chemoreflex sensitization during ALI recovery. Further understanding of the underlying mechanism will provide important information for the long-term goal of developing novel targeted therapeutic approaches to pulmonary diseases to improve clinical outcomes.
Keywords: acute respiratory distress syndrome; bleomycin; carotid body; chemoreceptors; glomus cells.
Copyright © 2023 Kamra, Karpuk, Zucker, Schultz and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bernard G. R., Artigas A., Brigham K. L., Carlet J., Falke K., Hudson L., et al. (1994). The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149 (3), 818–824. 10.1164/ajrccm.149.3.7509706 - DOI - PubMed
-
- Díaz H. S., Andrade D. C., Toledo C., Pereyra K. V., Schwarz K. G., Díaz-Jara E., et al. (2020). Episodic stimulation of central chemoreceptor neurons elicits disordered breathing and autonomic dysfunction in volume overload heart failure. Am. J. Physiology-Lung Cell. Mol. Physiology 318 (1), L27–L40. 10.1152/ajplung.00007.2019 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

