Nucleus tractus solitarii is required for the development and maintenance of phrenic and sympathetic long-term facilitation after acute intermittent hypoxia
- PMID: 36846346
- PMCID: PMC9949380
- DOI: 10.3389/fphys.2023.1120341
Nucleus tractus solitarii is required for the development and maintenance of phrenic and sympathetic long-term facilitation after acute intermittent hypoxia
Abstract
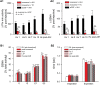
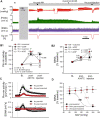
Exposure to acute intermittent hypoxia (AIH) induces prolonged increases (long term facilitation, LTF) in phrenic and sympathetic nerve activity (PhrNA, SNA) under basal conditions, and enhanced respiratory and sympathetic responses to hypoxia. The mechanisms and neurocircuitry involved are not fully defined. We tested the hypothesis that the nucleus tractus solitarii (nTS) is vital to augmentation of hypoxic responses and the initiation and maintenance of elevated phrenic (p) and splanchnic sympathetic (s) LTF following AIH. nTS neuronal activity was inhibited by nanoinjection of the GABAA receptor agonist muscimol before AIH exposure or after development of AIH-induced LTF. AIH but not sustained hypoxia induced pLTF and sLTF with maintained respiratory modulation of SSNA. nTS muscimol before AIH increased baseline SSNA with minor effects on PhrNA. nTS inhibition also markedly blunted hypoxic PhrNA and SSNA responses, and prevented altered sympathorespiratory coupling during hypoxia. Inhibiting nTS neuronal activity before AIH exposure also prevented the development of pLTF during AIH and the elevated SSNA after muscimol did not increase further during or following AIH exposure. Furthermore, nTS neuronal inhibition after the development of AIH-induced LTF substantially reversed but did not eliminate the facilitation of PhrNA. Together these findings demonstrate that mechanisms within the nTS are critical for initiation of pLTF during AIH. Moreover, ongoing nTS neuronal activity is required for full expression of sustained elevations in PhrNA following exposure to AIH although other regions likely also are important. Together, the data indicate that AIH-induced alterations within the nTS contribute to both the development and maintenance of pLTF.
Keywords: muscimol; peripheral chemoreflex; phrenic nerve activity; splanchnic sympathetic nerve activity; sympathorespiratory coupling.
Copyright © 2023 Ostrowski, Heesch, Kline and Hasser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
The development but not the maintenance of phrenic and sympathetic long-term facilitation after acute intermittent hypoxia requires nucleus tractus solitarii H2O2.Exp Neurol. 2025 Nov;393:115380. doi: 10.1016/j.expneurol.2025.115380. Epub 2025 Jul 16. Exp Neurol. 2025. PMID: 40680878
-
Hypothalamic PVN contributes to acute intermittent hypoxia-induced sympathetic but not phrenic long-term facilitation.J Appl Physiol (1985). 2018 May 1;124(5):1233-1243. doi: 10.1152/japplphysiol.00743.2017. Epub 2017 Dec 21. J Appl Physiol (1985). 2018. PMID: 29357503 Free PMC article.
-
Paraventricular nucleus projections to the nucleus tractus solitarii are essential for full expression of hypoxia-induced peripheral chemoreflex responses.J Physiol. 2023 Oct;601(19):4309-4336. doi: 10.1113/JP284907. Epub 2023 Aug 26. J Physiol. 2023. PMID: 37632733
-
Circulatory control of phrenic motor plasticity.Respir Physiol Neurobiol. 2019 Jul;265:19-23. doi: 10.1016/j.resp.2019.01.004. Epub 2019 Jan 11. Respir Physiol Neurobiol. 2019. PMID: 30639504 Free PMC article. Review.
-
NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation.J Physiol. 2009 May 1;587(Pt 9):1931-42. doi: 10.1113/jphysiol.2008.165597. Epub 2009 Feb 23. J Physiol. 2009. PMID: 19237427 Free PMC article. Review.
Cited by
-
Cardiac neuromodulation with acute intermittent hypoxia in rats with spinal cord injury.J Physiol. 2025 Mar;603(7):2139-2156. doi: 10.1113/JP287676. Epub 2025 Mar 22. J Physiol. 2025. PMID: 40120122 Free PMC article.
-
Partial reduction of parasympathetic nerve activity during sleep after endurance exercise under hypoxic conditions.Phys Act Nutr. 2025 Jun;29(2):35-40. doi: 10.20463/pan.2025.0012. Epub 2025 Jun 30. Phys Act Nutr. 2025. PMID: 40765070 Free PMC article.
-
Electroacupuncture Regulates Sympathetic Nerve Through the NTSGlu-RVLM Circuit to Relieve Spontaneous Pain in SNI Rats.CNS Neurosci Ther. 2025 Mar;31(3):e70327. doi: 10.1111/cns.70327. CNS Neurosci Ther. 2025. PMID: 40150822 Free PMC article.
-
Identification of multiple hypoxia-independent triggers of upper airway long-term facilitation in a rat model of upper airway motor plasticity.Physiol Rep. 2025 Mar;13(5):e70142. doi: 10.14814/phy2.70142. Physiol Rep. 2025. PMID: 40067835 Free PMC article.
-
Chronic intermittent hypoxia promotes glomerular hyperfiltration and potentiates hypoxia-evoked decreases in renal perfusion and PO2.Front Physiol. 2023 Jul 6;14:1235289. doi: 10.3389/fphys.2023.1235289. eCollection 2023. Front Physiol. 2023. PMID: 37485067 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

