New perspective of skeletal stem cells
- PMID: 36846511
- PMCID: PMC9947737
- DOI: 10.12336/biomatertransl.2022.04.007
New perspective of skeletal stem cells
Abstract
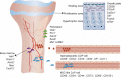
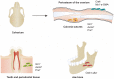
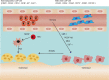
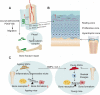
Tissue-resident stem cells are a group of stem cells distinguished by their capacity for self-renewal and multilineage differentiation capability with tissue specificity. Among these tissue-resident stem cells, skeletal stem cells (SSCs) were discovered in the growth plate region through a combination of cell surface markers and lineage tracing series. With the process of unravelling the anatomical variation of SSCs, researchers were also keen to investigate the developmental diversity outside the long bones, including in the sutures, craniofacial sites, and spinal regions. Recently, fluorescence-activated cell sorting, lineage tracing, and single-cell sequencing have been used to map lineage trajectories by studying SSCs with different spatiotemporal distributions. The SSC niche also plays a pivotal role in regulating SSC fate, such as cell-cell interactions mediated by multiple signalling pathways. This review focuses on discussing the spatial and temporal distribution of SSCs, and broadening our understanding of the diversity and plasticity of SSCs by summarizing the progress of research into SSCs in recent years.
Keywords: bone repair; endochondral ossification; growth plate; lineage tracing; skeletal stem cells.
Figures
Similar articles
-
Skeletal stem cells in bone development, homeostasis, and disease.Protein Cell. 2024 Jul 20;15(8):559-574. doi: 10.1093/procel/pwae008. Protein Cell. 2024. PMID: 38442300 Free PMC article. Review.
-
Insights into skeletal stem cells.Bone Res. 2022 Oct 19;10(1):61. doi: 10.1038/s41413-022-00235-8. Bone Res. 2022. PMID: 36261411 Free PMC article. Review.
-
Periosteal Skeletal Stem Cells and Their Response to Bone Injury.Front Cell Dev Biol. 2022 Mar 24;10:812094. doi: 10.3389/fcell.2022.812094. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399528 Free PMC article. Review.
-
Skeletal stem cells: origins, definitions, and functions in bone development and disease.Life Med. 2022 Dec 8;1(3):276-293. doi: 10.1093/lifemedi/lnac048. eCollection 2022 Dec. Life Med. 2022. PMID: 36811112 Free PMC article. Review.
-
Skeletal stem cells: a game changer of skeletal biology and regenerative medicine?Life Med. 2022 Sep 14;1(3):294-306. doi: 10.1093/lifemedi/lnac038. eCollection 2022 Dec. Life Med. 2022. PMID: 36811113 Free PMC article. Review.
Cited by
-
Bone-organ axes: bidirectional crosstalk.Mil Med Res. 2024 Jun 12;11(1):37. doi: 10.1186/s40779-024-00540-9. Mil Med Res. 2024. PMID: 38867330 Free PMC article. Review.
-
Novel carbon dots with dual Modulatory effects on the bone marrow and spleen as a potential therapeutic candidate for treating spinal cord injury.Bioact Mater. 2024 Dec 11;45:534-550. doi: 10.1016/j.bioactmat.2024.11.032. eCollection 2025 Mar. Bioact Mater. 2024. PMID: 39759534 Free PMC article.
-
Bone-derived factors mediate crosstalk between skeletal and extra-skeletal organs.Bone Res. 2025 Apr 30;13(1):49. doi: 10.1038/s41413-025-00424-1. Bone Res. 2025. PMID: 40307216 Free PMC article. Review.
-
Evaluation of Safety and Efficacy of Cell Therapy Based on Osteoblasts Derived from Umbilical Cord Mesenchymal Stem Cells for Osteonecrosis of the Femoral Head: Study Protocol for a Single-Center, Open-Label, Phase I Clinical Trial.Pharmaceuticals (Basel). 2024 Oct 13;17(10):1366. doi: 10.3390/ph17101366. Pharmaceuticals (Basel). 2024. PMID: 39459006 Free PMC article.
-
METTL3 inhibits BMSC apoptosis and facilitates osteonecrosis repair via an m6A-IGF2BP2-dependent mechanism.Heliyon. 2024 May 3;10(10):e30195. doi: 10.1016/j.heliyon.2024.e30195. eCollection 2024 May 30. Heliyon. 2024. PMID: 38784565 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
