Humoral response after BNT162b2 vaccine in Japanese hemodialysis patients
- PMID: 36846515
- PMCID: PMC9939857
- DOI: 10.1186/s41100-022-00452-1
Humoral response after BNT162b2 vaccine in Japanese hemodialysis patients
Abstract
Background: Hemodialysis patients are more likely to be severely affected if infected by COVID-19. Contributing factors include chronic kidney disease, old age, hypertension, type 2 diabetes, heart disease, and cerebrovascular disease. Therefore, action against COVID-19 for hemodialysis patients is an urgent issue. Vaccines are effective in preventing COVID 19 infection. In hemodialysis patients, however, responses to hepatitis B and influenza vaccines are reportedly weak. The BNT162b2 vaccine has shown an efficacy rate of about 95% in the general population, but as far as we know there are only several reports of efficacy data in hemodialysis patients in Japan.
Methods: We assessed serum anti-SARS-CoV-2 IgG antibody (Abbott SARS-CoV-2 IgG II Quan) in 185 hemodialysis patients and 109 health care workers. The exclusion criterion was positivity for SARS-CoV-2 IgG antibody before vaccination. Adverse reactions to BNT162b2 vaccine were evaluated through interviews.
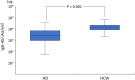
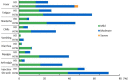
Results: Following vaccination, 97.6% of the hemodialysis group and 100% of the control group were positive for the anti-spike antibody. The median level of anti-spike antibody was 2,728.7 AU/mL (IQR, 1,024.2-7,688.2 AU/mL) in the hemodialysis group and 10,500 AU/ml (IQR, 9,346.1-2,4500 AU/mL) in the health care workers group. The factors involved in the low response to the BNT152b2 vaccine included old age, low BMI, low Cr index, low nPCR, low GNRI, low lymphocyte count, steroid administration, and complications related to blood disorders.
Conclusions: Humoral responses to BNT162b2 vaccine in hemodialysis patients are weaker than in a healthy control sample. Booster vaccination is necessary for hemodialysis patients, especially those showing a weak or non-response to the two-dose BNT162b2 vaccine.Trial registration UMIN, UMIN000047032. Registered 28 February 2022, https://center6.umin.ac.jp/cgi-bin/ctr/ctr_reg_rec.cgi.
Keywords: BNT162b2 vaccine; COVID-19 prevention; Japanese hemodialysis patients.
© The Author(s) 2023.
Conflict of interest statement
Competing interestsNo authors have conflicts to report.
Figures
Similar articles
-
Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study.Lancet Haematol. 2021 Aug;8(8):e583-e592. doi: 10.1016/S2352-3026(21)00169-1. Epub 2021 Jul 2. Lancet Haematol. 2021. PMID: 34224668 Free PMC article.
-
Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis.JAMA Netw Open. 2021 Sep 1;4(9):e2123622. doi: 10.1001/jamanetworkopen.2021.23622. JAMA Netw Open. 2021. PMID: 34473256 Free PMC article.
-
SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis.Am J Kidney Dis. 2022 Feb;79(2):185-192.e1. doi: 10.1053/j.ajkd.2021.08.005. Epub 2021 Sep 8. Am J Kidney Dis. 2022. PMID: 34508833 Free PMC article.
-
Factors associated with anti-SARS-CoV-2 spike antibody titers after a second BNT162b2 mRNA COVID-19 vaccination in Japanese hemodialysis patients.Clin Exp Nephrol. 2022 Sep;26(9):925-932. doi: 10.1007/s10157-022-02223-y. Epub 2022 Apr 15. Clin Exp Nephrol. 2022. PMID: 35426594 Free PMC article.
-
Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine.Front Immunol. 2022 Jan 31;13:827306. doi: 10.3389/fimmu.2022.827306. eCollection 2022. Front Immunol. 2022. PMID: 35173736 Free PMC article.
Cited by
-
Impact of COVID-19 Vaccination on Mortality and Clinical Outcomes in Hemodialysis Patients.Vaccines (Basel). 2024 Jul 19;12(7):799. doi: 10.3390/vaccines12070799. Vaccines (Basel). 2024. PMID: 39066437 Free PMC article.
-
Factors Associated with Neutralizing Antibody Responses following 2-Dose and 3rd Booster Monovalent COVID-19 Vaccination in Japanese People Living with HIV.Viruses. 2024 Apr 2;16(4):555. doi: 10.3390/v16040555. Viruses. 2024. PMID: 38675897 Free PMC article.
References
-
- World Health Organization (WHO). Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2022. Accessed 5 Oct 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous
