Comparison of Goal-Directed Fluid Therapy using LiDCOrapid System with Regular Fluid Therapy in Patients Undergoing Spine Surgery as a Randomised Clinical Trial
- PMID: 36846537
- PMCID: PMC9949010
- DOI: 10.2478/rjaic-2021-0001
Comparison of Goal-Directed Fluid Therapy using LiDCOrapid System with Regular Fluid Therapy in Patients Undergoing Spine Surgery as a Randomised Clinical Trial
Abstract
Background: Goal-directed fluid therapy (GDFT) is a new concept to describe the cardiac output (CO) and stroke volume variation to guide intravenous fluid administration during surgery. LiDCOrapid (LiDCO, Cardiac Sensor System, UK Company Regd 2736561, VAT Regd 672475708) is a minimally invasive monitor that estimates the responsiveness of CO versus fluid infusion. We intend to find whether GDFT using the LiDCOrapid system can decrease the volume of intraoperative fluid therapy and facilitate recovery in patients undergoing posterior fusion spine surgeries in comparison to regular fluid therapy.
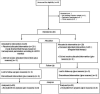
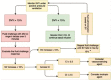
Methods: This study is a randomised clinical trial, and the design was parallel. Inclusion criteria for participants in this study were patients with comorbidities such as diabetes mellitus, hypertension, and ischemic heart disease undergoing spine surgery; exclusion criteria were patients with irregular heart rhythm or severe valvular heart disease. Forty patients with a previous history of medical comorbidities undergoing spine surgery were randomly and evenly assigned to receive either LiDCOrapid guided fluid therapy or regular fluid therapy. The volume of infused fluid was the primary outcome. The amount of bleeding, number of patients who needed packed red blood cell transfusion, base deficit, urine output, days of hospital length of stay and intensive care unit (ICU) admission, and time needed to start eating solids were monitored as secondary outcomes.
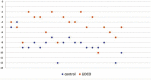
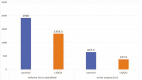
Results: The volume of infused crystalloid and urinary output in the LiDCO group was significantly lower than that of the control group (p = .001). Base deficit at the end of surgery was significantly better in the LiDCO group (p < .001). The duration of hospital length of stay in the LiDCO group was significantly shorter (p = .027), but the duration of ICU admission was not significantly different between the two groups.
Conclusion: Goal-directed fluid therapy using the LiDCOrapid system reduced the volume of intraoperative fluid therapy.
Keywords: Fluid therapy; LiDCOrapid; hemodynamics; spine surgery; stroke volume.
© 2021 Reza Shariat Moharari et al., published by Sciendo.
Conflict of interest statement
Conflict of interest: None.
Figures
Similar articles
-
Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy.J Clin Anesth. 2013 Sep;25(6):466-74. doi: 10.1016/j.jclinane.2013.04.010. Epub 2013 Aug 17. J Clin Anesth. 2013. PMID: 23965199
-
Goal-Directed Fluid Therapy Based on Stroke Volume Variation in Patients Undergoing Major Spine Surgery in the Prone Position: A Cohort Study.Spine (Phila Pa 1976). 2016 Sep 15;41(18):E1131-E1137. doi: 10.1097/BRS.0000000000001601. Spine (Phila Pa 1976). 2016. PMID: 27046635
-
Pleth variability index versus pulse pressure variation for intraoperative goal-directed fluid therapy in patients undergoing low-to-moderate risk abdominal surgery: a randomized controlled trial.BMC Anesthesiol. 2019 Mar 9;19(1):34. doi: 10.1186/s12871-019-0707-9. BMC Anesthesiol. 2019. PMID: 30851740 Free PMC article. Clinical Trial.
-
Current Commonly Used Dynamic Parameters and Monitoring Systems for Perioperative Goal-Directed Fluid Therapy: A Review.Yale J Biol Med. 2023 Mar 31;96(1):107-123. doi: 10.59249/JOAP6662. eCollection 2023 Mar. Yale J Biol Med. 2023. PMID: 37009197 Free PMC article. Review.
-
Intraoperative goal-directed fluid therapy in neurosurgical patients: A systematic review.Surg Neurol Int. 2024 Jul 5;15:233. doi: 10.25259/SNI_412_2024. eCollection 2024. Surg Neurol Int. 2024. PMID: 39108391 Free PMC article. Review.
Cited by
-
Index of Consciousness monitoring may effectively predict and prevent circulatory stress induced by endotracheal intubation under general anesthesia: a prospective randomized controlled trial.BMC Anesthesiol. 2024 Sep 6;24(1):316. doi: 10.1186/s12871-024-02701-8. BMC Anesthesiol. 2024. PMID: 39243003 Free PMC article. Clinical Trial.
References
-
- Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth. 2015;114(5):767–76. doi: 10.1093/bja/aeu452. Epub 2015/01/15. PubMed PMID: 25586725. - DOI - PubMed
-
- Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z. et al. Fluid challenges in intensive care: the FENICE study--a global inception cohort study. Intensive Care Med. 2015;41(9):1529–37. doi: 10.1007/s00134-015-3850-x. Epub 2015/07/15. PubMed PMID: 26162676; PubMed Central PMCID: PMCPMC4550653. - DOI - PMC - PubMed
-
- Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. Epub 2010/10/23. PubMed PMID: 20966436. - DOI - PubMed
-
- Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J. et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118(5):1052–61. doi: 10.1213/ane.0000000000000206. Epub 2014/05/02. PubMed PMID: 24781574. - DOI - PubMed
LinkOut - more resources
Full Text Sources
