Validating expression of beta cell maturation-associated genes in human pancreas development
- PMID: 36846594
- PMCID: PMC9945361
- DOI: 10.3389/fcell.2023.1103719
Validating expression of beta cell maturation-associated genes in human pancreas development
Abstract
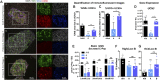
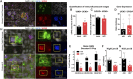
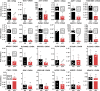
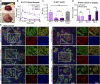
The identification of genes associated with human pancreatic beta cell maturation could stimulate a better understanding of normal human islet development and function, be informative for improving stem cell-derived islet (SC-islet) differentiation, and facilitate the sorting of more mature beta cells from a pool of differentiated cells. While several candidate factors to mark beta cell maturation have been identified, much of the data supporting these markers come from animal models or differentiated SC-islets. One such marker is Urocortin-3 (UCN3). In this study, we provide evidence that UCN3 is expressed in human fetal islets well before the acquisition of functional maturation. When SC-islets expressing significant levels of UCN3 were generated, the cells did not exhibit glucose-stimulated insulin secretion, indicating that UCN3 expression is not correlated with functional maturation in these cells. We utilized our tissue bank and SC-islet resources to test an array of other candidate maturation-associated genes, and identified CHGB, G6PC2, FAM159B, GLUT1, IAPP and ENTPD3 as markers with expression patterns that correlate developmentally with the onset of functional maturation in human beta cells. We also find that human beta cell expression of ERO1LB, HDAC9, KLF9, and ZNT8 does not change between fetal and adult stages.
Keywords: development; human; islets; maturation; stem cells.
Copyright © 2023 Tremmel, Mikat, Gupta, Mitchell, Curran, Menadue, Odorico and Sackett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

