The short-chain fatty acid butyrate exerts a specific effect on VE-cadherin phosphorylation and alters the integrity of aortic endothelial cells
- PMID: 36846596
- PMCID: PMC9944439
- DOI: 10.3389/fcell.2023.1076250
The short-chain fatty acid butyrate exerts a specific effect on VE-cadherin phosphorylation and alters the integrity of aortic endothelial cells
Abstract
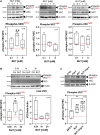
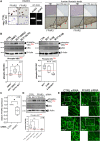
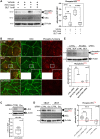
Short-chain fatty acids (SCFAs) like butyrate (BUT) largely influence vascular integrity and are closely associated with the onset and progression of cardiovascular diseases. However, their impact on vascular endothelial cadherin (VEC), a major vascular adhesion and signaling molecule, is largely unknown. Here, we explored the effect of the SCFA BUT on the phosphorylation of specific tyrosine residues of VEC (Y731, Y685, and Y658), which are reported to be critical for VEC regulation and vascular integrity. Moreover, we shed light on the signaling pathway engaged by BUT to affect the phosphorylation of VEC. Thereby, we used phospho-specific antibodies to evaluate the phosphorylation of VEC in response to the SCFA sodium butyrate in human aortic endothelial cells (HAOECs) and performed dextran assays to analyze the permeability of the EC monolayer. The role of c-Src and SCFA receptors FFAR2 and FFAR3 in the induction of VEC phosphorylation was analyzed using inhibitors and antagonists for c-Src family kinases and FFAR2/3, respectively, as well as by RNAi-mediated knockdown. Localization of VEC in response to BUT was assessed by fluorescence microscopy. BUT treatment of HAOEC resulted in the specific phosphorylation of Y731 at VEC with minor effects on Y685 and Y658. Thereby, BUT engages FFAR3, FFAR2, and c-Src kinase to induce phosphorylation of VEC. VEC phosphorylation correlated with enhanced endothelial permeability and c-Src-dependent remodeling of junctional VEC. Our data suggest that BUT, an SCFA and gut microbiota-derived metabolite, impacts vascular integrity by targeting VEC phosphorylation with potential impact on the pathophysiology and therapy of vascular diseases.
Keywords: VE-cadherin; aortic endothelial cell; butyrate; c-src; free fatty acid receptor (FFAR); permeability; phosphorylation; short-chain fatty acid (SCFA).
Copyright © 2023 Guo, Terhorst, Stammer, Ibrahim, Oberhuber and Eierhoff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
VE-cadherin shedding in vitro and in patients with aortic aneurysm and dissection.Sci Rep. 2024 Nov 5;14(1):26743. doi: 10.1038/s41598-024-77940-3. Sci Rep. 2024. PMID: 39501015 Free PMC article.
-
Chlamydia pneumoniae infection promotes monocyte transendothelial migration by increasing vascular endothelial cell permeability via the tyrosine phosphorylation of VE-cadherin.Biochem Biophys Res Commun. 2018 Mar 4;497(2):742-748. doi: 10.1016/j.bbrc.2018.02.145. Epub 2018 Feb 17. Biochem Biophys Res Commun. 2018. PMID: 29462613
-
Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site.Oncogene. 2007 Feb 15;26(7):1067-77. doi: 10.1038/sj.onc.1209855. Epub 2006 Aug 14. Oncogene. 2007. PMID: 16909109
-
Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View.Biomedicines. 2020 Jun 8;8(6):154. doi: 10.3390/biomedicines8060154. Biomedicines. 2020. PMID: 32521775 Free PMC article. Review.
-
Short-chain fatty acids: possible regulators of insulin secretion.Mol Cell Biochem. 2023 Mar;478(3):517-530. doi: 10.1007/s11010-022-04528-8. Epub 2022 Aug 9. Mol Cell Biochem. 2023. PMID: 35943655 Review.
Cited by
-
VE-cadherin shedding in vitro and in patients with aortic aneurysm and dissection.Sci Rep. 2024 Nov 5;14(1):26743. doi: 10.1038/s41598-024-77940-3. Sci Rep. 2024. PMID: 39501015 Free PMC article.
-
NAMPT Impairs Vascular Permeability in Periodontitis by Influencing FASN-mediated Lipogenesis.Int J Biol Sci. 2025 Mar 31;21(6):2707-2724. doi: 10.7150/ijbs.104485. eCollection 2025. Int J Biol Sci. 2025. PMID: 40303304 Free PMC article.
-
Association between the dietary index for gut microbiota and all-cause/cardiovascular mortality in patients with metabolic dysfunction-associated steatotic liver disease.Diabetol Metab Syndr. 2025 Jul 11;17(1):263. doi: 10.1186/s13098-025-01846-x. Diabetol Metab Syndr. 2025. PMID: 40646653 Free PMC article.
-
Circadian rhythms of gut microbiota and plaque vulnerability: mechanisms and chrono-microbiota modulation interventions.Gut Microbes. 2025 Dec;17(1):2532703. doi: 10.1080/19490976.2025.2532703. Epub 2025 Jul 12. Gut Microbes. 2025. PMID: 40650475 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

