Is the proximal tubule the focus of tubulointerstitial fibrosis?
- PMID: 36846656
- PMCID: PMC9950842
- DOI: 10.1016/j.heliyon.2023.e13508
Is the proximal tubule the focus of tubulointerstitial fibrosis?
Abstract
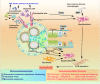
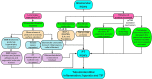
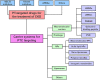
Tubulointerstitial fibrosis (TIF), a common end result of almost all progressive chronic kidney diseases (CKD), is also the best predictor of kidney survival. Almost all cells in the kidney are involved in the progression of TIF. Myofibroblasts, the primary producers of extracellular matrix, have previously received a great deal of attention; however, a large body of emerging evidence reveals that proximal tubule (PT) plays a central role in TIF progression. In response to injury, renal tubular epithelial cells (TECs) transform into inflammatory and fibroblastic cells, producing various bioactive molecules that drive interstitial inflammation and fibrosis. Here we reviewed the increasing evidence for the key role of the PT in promoting TIF in tubulointerstitial and glomerular injury and discussed the therapeutic targets and carrier systems involving the PT that holds particular promise for treating patients with fibrotic nephropathy.
Keywords: Chronic kidney disease; Extracellular matrix; Proximal tubule; Tubulointerstitial fibrosis.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Renal fibrosis: Primacy of the proximal tubule.Matrix Biol. 2018 Aug;68-69:248-262. doi: 10.1016/j.matbio.2018.02.006. Epub 2018 Feb 6. Matrix Biol. 2018. PMID: 29425694 Free PMC article. Review.
-
Loss of the Protein Cystathionine β-Synthase During Kidney Injury Promotes Renal Tubulointerstitial Fibrosis.Kidney Blood Press Res. 2017;42(3):428-443. doi: 10.1159/000479295. Epub 2017 Jul 27. Kidney Blood Press Res. 2017. PMID: 28750410
-
Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis.FASEB J. 2017 Oct;31(10):4407-4421. doi: 10.1096/fj.201601359RR. Epub 2017 Jun 16. FASEB J. 2017. PMID: 28626027 Free PMC article.
-
Different Patterns of Kidney Fibrosis Are Indicative of Injury to Distinct Renal Compartments.Cells. 2021 Aug 6;10(8):2014. doi: 10.3390/cells10082014. Cells. 2021. PMID: 34440782 Free PMC article.
-
Role of tubule epithelial cells in the pathogenesis of tubulointerstitial fibrosis induced by glomerular disease.Curr Opin Nephrol Hypertens. 1998 Sep;7(5):525-30. doi: 10.1097/00041552-199809000-00007. Curr Opin Nephrol Hypertens. 1998. PMID: 9818199 Review.
Cited by
-
JiangyaTongluo decoction ameliorates tubulointerstitial fibrosis via regulating the SIRT1/PGC-1α/mitophagy axis in hypertensive nephropathy.Front Pharmacol. 2024 Dec 12;15:1491315. doi: 10.3389/fphar.2024.1491315. eCollection 2024. Front Pharmacol. 2024. PMID: 39726785 Free PMC article.
-
Modified Hu-Lu-Ba-Wan Alleviates Early-Stage Diabetic Kidney Disease via Inhibiting Interleukin-17A in Mice.Chin J Integr Med. 2025 Jun;31(6):506-517. doi: 10.1007/s11655-024-3919-x. Epub 2025 Jan 7. Chin J Integr Med. 2025. PMID: 39762500
-
Claudin-2 Mediates the Proximal Tubular Epithelial Cell-Fibroblast Crosstalk via Paracrine CTGF.Diabetes Metab Syndr Obes. 2024 Jan 3;17:55-73. doi: 10.2147/DMSO.S432173. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38192494 Free PMC article.
-
Mechanism of Astragaloside IV in Treatment of Renal Tubulointerstitial Fibrosis.Chin J Integr Med. 2025 May;31(5):474-480. doi: 10.1007/s11655-024-3805-6. Epub 2024 Jun 8. Chin J Integr Med. 2025. PMID: 38850482 Review.
-
How PPAR-alpha mediated inflammation may affect the pathophysiology of chronic kidney disease.Curr Res Physiol. 2024 Nov 14;8:100133. doi: 10.1016/j.crphys.2024.100133. eCollection 2025. Curr Res Physiol. 2024. PMID: 39665027 Free PMC article. Review.
References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. - PMC - PubMed
-
- Zeisberg M., Neilson E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010;21(11):1819–1834. - PubMed
-
- Djudjaj S., Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Aspect. Med. 2019;65:16–36. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

