A Highly Active and Selective Zirconium-Based Catalyst System for the Industrial Production of Poly(lactic acid)
- PMID: 36846823
- PMCID: PMC9942235
- DOI: 10.1021/acscatal.2c05690
A Highly Active and Selective Zirconium-Based Catalyst System for the Industrial Production of Poly(lactic acid)
Abstract
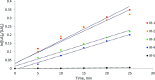
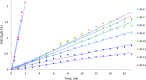
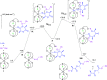
The biodegradable, aliphatic polyester poly(lactic acid), PLA, is a leading bio-based alternative to petrochemical-derived plastic materials across a range of applications. Widely reported in the available literature as a benchmark for PLA production via the bulk ring-opening polymerization of lactides is the use of divalent tin catalysts, and particularly tin(II) bis(2-ethylhexanoate). We present an alternative zirconium-based system that combines an inexpensive Group IV metal with the robustness, high activity, control, and designed compatibility with existing facilities and processes, that are required for industrial use. We have carried out a comprehensive kinetic study and applied a combined experimental and theoretical approach to understanding the mechanism by which the polymerization of lactide proceeds in the presence of this system. In the laboratory-scale (20 g) polymerization of recrystallized racemic d,l-lactide (rac-lactide), we have measured catalyst turnover frequencies up to at least 56,000 h-1, and confirmed the reported protocols' resistance toward undesirable epimerization, transesterification, and chain scission processes, deleterious to the properties of the polymer product. Further optimization and scale-up under industrial conditions have confirmed the relevance of the catalytic protocol to the commercial production of melt-polymerized PLA. We were able to undertake the efficient preparation of high-molecular-weight PLA on the 500-2000 g scale, via the selective and well-controlled polymerization of commercial polymer-grade l-lactide under challenging, industrially relevant conditions, and at metal concentrations as low as 8-12 ppm Zr by weight ([Zr] = 1.3 × 10-3 to 1.9 × 10-3 mol %). Under those conditions, a catalyst turnover number of at least 60,000 was attained, and the activity of the catalyst was comparable to that of tin(II) bis(2-ethylhexanoate).
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
N-Heterocyclic carbene iron complexes catalyze the ring-opening polymerization of lactide.Catal Sci Technol. 2022 Jan 5;12(3):996-1004. doi: 10.1039/d1cy02143e. eCollection 2022 Feb 7. Catal Sci Technol. 2022. PMID: 35222940 Free PMC article.
-
Indium Catalysts for Ring Opening Polymerization: Exploring the Importance of Catalyst Aggregation.Acc Chem Res. 2017 Nov 21;50(11):2861-2869. doi: 10.1021/acs.accounts.7b00447. Epub 2017 Oct 31. Acc Chem Res. 2017. PMID: 29087695 Review.
-
Group IV complexes containing the benzotriazole phenoxide ligand as catalysts for the ring-opening polymerization of lactides, epoxides and as precatalysts for the polymerization of ethylene.Dalton Trans. 2013 Dec 14;42(46):16412-27. doi: 10.1039/c3dt52065j. Dalton Trans. 2013. PMID: 24071827
-
New Kids in Lactide Polymerization: Highly Active and Robust Iron Guanidine Complexes as Superior Catalysts.ChemSusChem. 2019 May 21;12(10):2161-2165. doi: 10.1002/cssc.201900481. Epub 2019 Mar 19. ChemSusChem. 2019. PMID: 30811863
-
Robust Guanidine Metal Catalysts for the Ring-Opening Polymerization of Lactide under Industrially Relevant Conditions.Chempluschem. 2020 May;85(5):1044-1052. doi: 10.1002/cplu.202000252. Chempluschem. 2020. PMID: 32449840 Review.
Cited by
-
Simple divalent metal salts as robust and efficient initiators for the ring-opening polymerisation of rac-lactide.RSC Adv. 2024 Nov 29;14(51):38079-38084. doi: 10.1039/d4ra07747d. eCollection 2024 Nov 25. RSC Adv. 2024. PMID: 39619809 Free PMC article.
-
Diversity in Zwitterionic Metal Ammonium Tris(phenolate)s for the Controlled Immortal Polymerization of Lactide: Dramatic Activity Enhancement and Mechanistic Insight on Expansion beyond Zirconium.ACS Catal. 2025 May 14;15(11):9130-9149. doi: 10.1021/acscatal.5c01857. eCollection 2025 Jun 6. ACS Catal. 2025. PMID: 40502968 Free PMC article.
-
Ti and Zr complexes bearing guanidine-phenolate ligands: coordination chemistry and polymerization studies.RSC Adv. 2024 Aug 16;14(35):25889-25899. doi: 10.1039/d4ra05146g. eCollection 2024 Aug 12. RSC Adv. 2024. PMID: 39156754 Free PMC article.
-
Medical applications and prospects of polylactic acid materials.iScience. 2024 Dec 1;27(12):111512. doi: 10.1016/j.isci.2024.111512. eCollection 2024 Dec 20. iScience. 2024. PMID: 39759018 Free PMC article. Review.
-
Coordination of ε-Caprolactone to a Cationic Niobium(V) Alkoxide Complex: Fundamental Insight into Ring-Opening Polymerization via Coordination-Insertion.Inorg Chem. 2023 Sep 25;62(38):15688-15699. doi: 10.1021/acs.inorgchem.3c02491. Epub 2023 Sep 11. Inorg Chem. 2023. PMID: 37695575 Free PMC article.
References
-
- Rabnawaz M.; Wyman I.; Auras R.; Cheng S. A Roadmap towards Green Packaging: The Current Status and Future Outlook for Polyesters in the Packaging Industry. Green Chem. 2017, 19 (20), 4737–4753. 10.1039/C7GC02521A. - DOI
-
- Rasal R. M.; Janorkar A. V.; Hirt D. E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35 (3), 338–356. 10.1016/j.progpolymsci.2009.12.003. - DOI
-
- Azor L.; Bailly C.; Brelot L.; Henry M.; Mobian P.; Dagorne S. Stereoselective Synthesis of Biphenolate/Binaphtolate Titanate and Zirconate Alkoxide Species: Structural Characterization and Use in the Controlled ROP of Lactide. Inorg. Chem. 2012, 51 (20), 10876–10883. 10.1021/ic301352t. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
