The Prevention of Inflammation and the Maintenance of Iron and Hepcidin Homeostasis in the Gut, Liver, and Brain Pathologies
- PMID: 36846996
- PMCID: PMC10116142
- DOI: 10.3233/JAD-220224
The Prevention of Inflammation and the Maintenance of Iron and Hepcidin Homeostasis in the Gut, Liver, and Brain Pathologies
Abstract
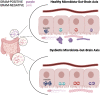
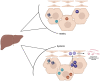
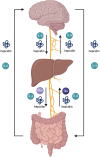
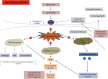
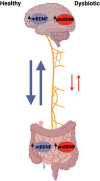
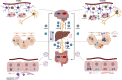
The human gut microbiome consists of a variety of microorganisms that inhabit the intestinal tract. This flora has recently been shown to play an important role in human disease. The crosstalk between the gut and brain axis has been investigated through hepcidin, derived from both hepatocytes and dendritic cells. Hepcidin could potentially play an anti-inflammatory role in the process of gut dysbiosis through a means of either a localized approach of nutritional immunity, or a systemic approach. Like hepcidin, mBDNF and IL-6 are part of the gut-brain axis: gut microbiota affects their levels of expression, and this relationship is thought to play a role in cognitive function and decline, which could ultimately lead to a number of neurodegenerative diseases such as Alzheimer's disease. This review will focus on the interplay between gut dysbiosis and the crosstalk between the gut, liver, and brain and how this is mediated by hepcidin through different mechanisms including the vagus nerve and several different biomolecules. This overview will also focus on the gut microbiota-induced dysbiotic state on a systemic level, and how gut dysbiosis can contribute to beginnings and the progression of Alzheimer's disease and neuroinflammation.
Keywords: Brain; TMAO; Western diet; dysbiosis; hepcidin; high fat-high sugar diet; intestines; mBDNF; vagus nerve.
Conflict of interest statement
The authors have no conflicts of interest to disclose with this work.
Figures


References
-
- Gaines S, Williamson AJ, Hyman N, Kandel J (2018) How the microbiome is shaping our understanding of cancer biology and its treatment. Semin Colon Rectal Surg 29, 12–16.
-
- Akbar N, Khan NA, Muhammad JS, Siddiqui R (2022) The role of gut microbiome in cancer genesis and cancer prevention. Health Sci Rev 2, 100010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

