Mercaptopurine for the Treatment of Ulcerative Colitis: A Randomized Placebo-Controlled Trial
- PMID: 36847130
- PMCID: PMC10394500
- DOI: 10.1093/ecco-jcc/jjad022
Mercaptopurine for the Treatment of Ulcerative Colitis: A Randomized Placebo-Controlled Trial
Abstract
Background and aims: Scepticism about the efficacy of thiopurines for ulcerative colitis [UC] is rising. This study aimed to evaluate mercaptopurine treatment for UC.
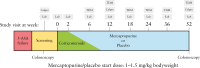
Methods: In this prospective, randomized, double-blind, placebo-controlled trial, patients with active UC, despite treatment with 5-aminosalicylates [5-ASA], were randomized for therapeutic drug monitoring [TDM]-guided mercaptopurine treatment or placebo for 52 weeks. Corticosteroids were given in the first 8 weeks and 5-ASA was continued. Proactive metabolite-based mercaptopurine and placebo dose adjustments were applied from week 6 onwards by unblinded clinicians. The primary endpoint was corticosteroid-free clinical remission and endoscopic improvement [total Mayo score ≤2 points and no item >1] at week 52 in an intention-to-treat analysis.
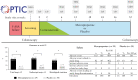
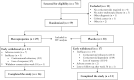
Results: Between December 2016 and April 2021, 70 patients were screened and 59 were randomized at six centres. In the mercaptopurine group, 16/29 [55.2%] patients completed the 52-week study, compared to 13/30 [43.3%] on placebo. The primary endpoint was achieved by 14/29 [48.3%] patients on mercaptopurine and 3/30 [10%] receiving placebo (Δ = 38.3%, 95% confidence interval [CI] 17.1-59.4, p = 0.002). Adverse events occurred more frequently with mercaptopurine [808.8 per 100 patient-years] compared to placebo [501.4 per 100 patient-years]. Five serious adverse events occurred, four on mercaptopurine and one on placebo. TDM-based dose adjustments were executed in 22/29 [75.9%] patients, leading to lower mercaptopurine doses at week 52 compared to baseline.
Conclusions: Optimized mercaptopurine treatment was superior to placebo in achieving clinical, endoscopic and histological outcomes at 1 year following corticosteroid induction treatment in UC patients. More adverse events occurred in the mercaptopurine group.
Keywords: Ulcerative colitis; immunomodulators; randomized controlled trial; therapeutic drug monitoring.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
ML: Abbvie, Alimentiv, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen-Cilag, Medtronic, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW. AV, SG, AM, NM, EC, SR, EvdZ, MDi: have no conflicts of interest. MDu: reports advisory fees from Echo Pharma and Robarts Clinical Trials, Inc., speaker fees from Janssen, Merck & Co., Inc., Pfizer, Takeda and Tillotts Pharma, and non-financial support from Dr. Falk Pharm. AvB: served as speaker, adviser and/or principal investigator for AbbVie, Arandal, Arena Pharmaceuticals/Pfizer, Celgene, Ferring, Galapagos/Gilead, Janssen/Johnson and Johnson, Merck Sharpe & Dohme, Pfizer, Receptos, Roche, Takeda, TEVA, Bristol Myers Squibb, and received research grants from TEVA, Eurostars funding, ZonMW, and Pfizer. JJ: has served on advisory boards, or as speaker or consultant for Abbvie, Amgen, Ferring, Fresenius, Janssen, MSD, Pfizer, Takeda. DvA: has served on advisory boards, or as speaker or consultant for Ferring, DrFalk, Takeda, Janssen, Galapagos. He has received research grants from Noordwest Academie, Janssen and DrFalk. RW: has participated in advisory board or as speaker for Jansen, Abbvie and Pfizer. NdB: has served as a speaker for AbbVie and MSD and has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received a [unrestricted] research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda. All outside the submitted work. GDH: Consultancy for Abbvie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Meiers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Pfizer, Immunic, Johnson and Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus laboratories, Prometheus Biosciences, Progenity, Protagonist. Speaker’s bureau for Abbvie, Arena, Galapagos, Gilead, Pfizer, BMS, Takeda.
Figures



Comment in
-
Thiopurines for Ulcerative Colitis: Old Favourite or Old Hat?J Crohns Colitis. 2023 Jul 5;17(7):1029-1030. doi: 10.1093/ecco-jcc/jjad062. J Crohns Colitis. 2023. PMID: 37141302 No abstract available.
References
-
- de Boer NKH, Peyrin-Biroulet L, Jharap B, et al. . Thiopurines in inflammatory bowel disease: new findings and perspectives. J Crohns Colitis 2018;12:610–20. - PubMed
-
- Jeuring SFG, van den Heuvel TRA, Zeegers MP, et al. . Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age—an increasing distinct entity? Inflamm Bowel Dis 2016;22:1425–34. - PubMed
-
- Chaparro M, Ordás I, Cabré E, et al. . Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 2013;19:1404–10. - PubMed
-
- Jharap B, Seinen ML, de Boer NK, et al. . Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis 2010;16:1541–9. - PubMed
-
- Gisbert JP, Linares PM, Mcnicholl AG, Maté J, Gomollón FM. The efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther 2009;30:126–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

