A Beta Strain-Based Spike Glycoprotein Vaccine Candidate Induces Broad Neutralization and Protection against SARS-CoV-2 Variants of Concern
- PMID: 36847495
- PMCID: PMC10100794
- DOI: 10.1128/spectrum.02687-22
A Beta Strain-Based Spike Glycoprotein Vaccine Candidate Induces Broad Neutralization and Protection against SARS-CoV-2 Variants of Concern
Abstract
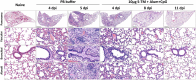
The coronavirus disease 2019 (COVID-19) pandemic is still ongoing. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) are circulating worldwide, making it resistant to existing vaccines and antiviral drugs. Therefore, the evaluation of variant-based expanded spectrum vaccines to optimize the immune response and provide broad protectiveness is very important. In this study, we expressed spike trimer protein (S-TM) based on the Beta variant in a GMP-grade workshop using CHO cells. Mice were immunized twice with S-TM protein combined with aluminum hydroxide (Al) and CpG Oligonucleotides (CpG) adjuvant to evaluate its safety and efficacy. BALB/c immunized with S-TM + Al + CpG induced high neutralizing antibody titers against the Wuhan-Hu-1 strain (wild-type, WT), the Beta and Delta variants, and even the Omicron variant. In addition, compared with the S-TM + Al group, the S-TM + Al + CpG group effectively induced a stronger Th1-biased cell immune response in mice. Furthermore, after the second immunization, H11-K18 hACE2 mice were well protected from challenge with the SARS-CoV-2 Beta strain, with a 100% survival rate. The virus load and pathological lesions in the lungs were significantly reduced, and no virus was detected in mouse brain tissue. Our vaccine candidate is practical and effective for current SARS-CoV-2 VOCs, which will support its further clinical development for potential sequential immune and primary immunization. IMPORTANCE Continuous emergence of adaptive mutations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to challenge the use and development of existing vaccines and drugs. The value of variant-based vaccines that are capable of inducing a higher and broader protection immune response against SARS-CoV-2 variants is currently being evaluated. This article shows that a recombinant prefusion spike protein based on a Beta variant was highly immunogenic and could induced a stronger Th1-biased cell immune response in mice and was effectively protective against challenge with the SARS-CoV-2 Beta variant. Importantly, this Beta-based SARS-CoV-2 vaccine could also offer a robust humoral immune response with effectively broad neutralization ability against the wild type and different variants of concern (VOCs): the Beta, Delta, and Omicron BA.1 variants. To date, the vaccine described here has been produced in a pilot scale (200L), and the development, filling process, and toxicological safety evaluation have also been completed, which provides a timely response to the emerging SARS-CoV-2 variants and vaccine development.
Keywords: SARS-CoV-2; broad neutralization; spike glycoprotein; variant-based vaccine; variants of concern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
A mosaic-type trimeric RBD-based COVID-19 vaccine candidate induces potent neutralization against Omicron and other SARS-CoV-2 variants.Elife. 2022 Aug 25;11:e78633. doi: 10.7554/eLife.78633. Elife. 2022. PMID: 36004719 Free PMC article.
-
Preclinical immune efficacy against SARS-CoV-2 beta B.1.351 variant by MVA-based vaccine candidates.Front Immunol. 2023 Dec 12;14:1264323. doi: 10.3389/fimmu.2023.1264323. eCollection 2023. Front Immunol. 2023. PMID: 38155964 Free PMC article.
-
A Synthetic SARS-CoV-2-Derived T-Cell and B-Cell Peptide Cocktail Elicits Full Protection against Lethal Omicron BA.1 Infection in H11-K18-hACE2 Mice.Microbiol Spectr. 2023 Mar 13;11(2):e0419422. doi: 10.1128/spectrum.04194-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36912685 Free PMC article.
-
The Development of Epitope-Based Recombinant Protein Vaccines against SARS-CoV-2.AAPS J. 2024 Aug 13;26(5):93. doi: 10.1208/s12248-024-00963-1. AAPS J. 2024. PMID: 39138686 Review.
-
Variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Vaccine Effectiveness.Vaccines (Basel). 2022 Oct 19;10(10):1751. doi: 10.3390/vaccines10101751. Vaccines (Basel). 2022. PMID: 36298616 Free PMC article. Review.
Cited by
-
SARS-CoV-2 spike-FLIPr fusion protein plus lipidated FLIPr protects against various SARS-CoV-2 variants in hamsters.J Virol. 2024 Feb 20;98(2):e0154623. doi: 10.1128/jvi.01546-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299865 Free PMC article.
References
-
- Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, Sominsky LA, Clark JJ, Adelsberg DC, Bielak DA, Gonzalez-Reiche AS, Dambrauskas N, Vigdorovich V, Alburquerque B, Amoako AA, Banu R, Beach KF, Bermúdez-González MC, Cai GY, Ceglia I, Cognigni C, Farrugia K, Gleason CR, van de Guchte A, Kleiner G, Khalil Z, Lyttle N, Mendez WA, Mulder LCF, Oostenink A, Rooker A, Salimbangon AT, Saksena M, Paniz-Mondolfi AE, Polanco J, Srivastava K, Sather DN, Sordillo EM, Bajic G, van Bakel H, Simon V, Krammer F, PSP-PARIS Study Group . 2022. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 602:682–688. doi:10.1038/d41586-021-03846-z. - DOI - PubMed
-
- Gupta SL, Mantus G, Manning KE, Ellis M, Patel M, Ciric CR, Lu A, Turner JS, O’Halloran JA, Presti RM, Joshi DJ, Ellebedy AH, Anderson EJ, Rostad CA, Suthar MS, Wrammert J. 2022. Loss of Pfizer (BNT162b2) vaccine-induced antibody responses against the SARS-CoV-2 Omicron variant in adolescents and adults. J Virol 96:e0058222. doi:10.1128/jvi.00582-22. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

