Intratympanic corticosteroids for Ménière's disease
- PMID: 36847608
- PMCID: PMC9969957
- DOI: 10.1002/14651858.CD015245.pub2
Intratympanic corticosteroids for Ménière's disease
Abstract
Background: Ménière's disease is a condition that causes recurrent episodes of vertigo, associated with hearing loss and tinnitus. Corticosteroids are sometimes administered directly into the middle ear to treat this condition (through the tympanic membrane). The underlying cause of Ménière's disease is unknown, as is the way in which this treatment may work. The efficacy of this intervention in preventing vertigo attacks, and their associated symptoms, is currently unclear.
Objectives: To evaluate the benefits and harms of intratympanic corticosteroids versus placebo or no treatment in people with Ménière's disease.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 14 September 2022.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs in adults with a diagnosis of Ménière's disease comparing intratympanic corticosteroids with either placebo or no treatment. We excluded studies with follow-up of less than three months, or with a cross-over design (unless data from the first phase of the study could be identified). DATA COLLECTION AND ANALYSIS: We used standard Cochrane methods. Our primary outcomes were: 1) improvement in vertigo (assessed as a dichotomous outcome - improved or not improved), 2) change in vertigo (assessed as a continuous outcome, with a score on a numerical scale) and 3) serious adverse events. Our secondary outcomes were: 4) disease-specific health-related quality of life, 5) change in hearing, 6) change in tinnitus and 7) other adverse effects (including tympanic membrane perforation). We considered outcomes reported at three time points: 3 to < 6 months, 6 to ≤ 12 months and > 12 months. We used GRADE to assess the certainty of evidence for each outcome. MAIN RESULTS: We included 10 studies with a total of 952 participants. All studies used the corticosteroid dexamethasone, with doses ranging from approximately 2 mg to 12 mg. Improvement in vertigo Intratympanic corticosteroids may make little or no difference to the number of people who report an improvement in their vertigo at 6 to ≤ 12 months follow-up (intratympanic corticosteroids 96.8%, placebo 96.6%, risk ratio (RR) 1.00, 95% confidence interval (CI) 0.92 to 1.10; 2 studies; 60 participants; low-certainty evidence) or at more than 12 months follow-up (intratympanic corticosteroids 100%, placebo 96.3%; RR 1.03, 95% CI 0.87 to 1.23; 2 studies; 58 participants; low-certainty evidence). However, we note the large improvement in the placebo group for these trials, which causes challenges in interpreting these results. Change in vertigo Assessed with a global score One study (44 participants) assessed the change in vertigo at 3 to < 6 months using a global score, which considered the frequency, duration and severity of vertigo. This is a single, small study and the certainty of the evidence was very low. We are unable to draw meaningful conclusions from the numerical results. Assessed by frequency of vertigo Three studies (304 participants) assessed the change in frequency of vertigo episodes at 3 to < 6 months. Intratympanic corticosteroids may slightly reduce the frequency of vertigo episodes. The proportion of days affected by vertigo was 0.05 lower (absolute difference -5%) in those receiving intratympanic corticosteroids (95% CI -0.07 to -0.02; 3 studies; 472 participants; low-certainty evidence). This is equivalent to a difference of approximately 1.5 days fewer per month affected by vertigo in the corticosteroid group (with the control group having vertigo on approximately 2.5 to 3.5 days per month at the end of follow-up, and those receiving corticosteroids having vertigo on approximately 1 to 2 days per month). However, this result should be interpreted with caution - we are aware of unpublished data at this time point in which corticosteroids failed to show a benefit over placebo. One study also assessed the change in frequency of vertigo at 6 to ≤ 12 months and > 12 months follow-up. However, this is a single, small study and the certainty of the evidence was very low. Therefore, we are unable to draw meaningful conclusions from the numerical results. Serious adverse events Four studies reported this outcome. There may be little or no effect on the occurrence of serious adverse events with intratympanic corticosteroids, but the evidence is very uncertain (intratympanic corticosteroids 3.0%, placebo 4.4%; RR 0.64, 95% CI 0.22 to 1.85; 4 studies; 500 participants; very low-certainty evidence).
Authors' conclusions: The evidence for intratympanic corticosteroids in the treatment of Ménière's disease is uncertain. There are relatively few published RCTs, which all consider the same type of corticosteroid (dexamethasone). We also have concerns about publication bias in this area, with the identification of two large RCTs that remain unpublished. The evidence comparing intratympanic corticosteroids to placebo or no treatment is therefore all low- or very low-certainty. This means that we have very low confidence that the effects reported are accurate estimates of the true effect of these interventions. Consensus on the appropriate outcomes to measure in studies of Ménière's disease is needed (i.e. a core outcome set) in order to guide future studies in this area, and enable meta-analysis of the results. This must include appropriate consideration of the potential harms of treatment, as well as the benefits. Finally, we would also highlight the responsibility that trialists have to ensure results are available, regardless of the outcome of their study.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Katie Webster: none known.
Ambrose Lee: none known.
Kevin Galbraith: none known.
Natasha A Harrington‐Benton: Natasha Harrington‐Benton is the Director of the Ménière's Society, a national charity supporting people with vestibular conditions. The Ménière's Society supports research in various ways, including distributing surveys and/or providing grant funding for projects studying vestibular conditions. Some of the studies they have previously funded may be included in the review. They do not carry out the research themselves and are not directly involved in projects.
Owen Judd: none known.
Diego Kaski: none known.
Otto R Maarsingh: none known.
Samuel MacKeith: Samuel MacKeith is the Assistant Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. He sees patients with Ménière's disease in his NHS and private practice and is the co‐director of a company providing private vestibular function testing services.
Jaydip Ray: none known.
Vincent A Van Vugt: none known.
Brian Westerberg: none known.
Martin J Burton: Martin Burton undertook private practice until March 2020 and saw some patients with Ménière's disease. He is the Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Figures
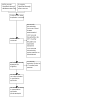
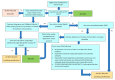

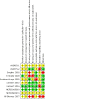











Update of
- doi: 10.1002/14651858.CD015245
References
References to studies included in this review
AVERTS‐1 {published data only}
-
- Lambert PR, Silverstein H, LeBel C, Bishop K. OTO-104 in Meniere's disease patients: phase 3 results. Otolaryngology - Head and Neck Surgery 2017;157 (1 Suppl 1):122-3.
-
- NCT02612337. A prospective, randomized, double-blind, placebo-controlled, multicenter, phase 3 study of OTO-104 given as a single intratympanic injection in subjects with unilateral Meniere's disease (NCT02612337). https://clinicaltrials.gov/ct2/show/NCT02612337 (first received 23 November 2015).
-
- Otonomy Reports Results for AVERTS-1 Phase 3 Trial for OTIVIDEX™ in Patients with Ménière's Disease. https://investors.otonomy.com/news-releases/news-release-details/otonomy....
AVERTS‐2 {published data only}
-
- A prospective, randomized, double-blind, placebo-controlled, multicentre, phase 3 efficacy and safety study of OTO-104 given as a single intratympanic injection in subjects with unilateral Ménière's disease. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-00449... 2021.
-
- Phillips JS, Gurkov R, Bishop K. AVERTS-2 phase 3 results of Otividex in Meniere disease. Otolaryngology - Head and Neck Surgery 2018;159(1 Suppl 1):P120.
-
- Study of OTO-104 in subjects with unilateral Meniere's disease (NCT02717442). https://clinicaltrials.gov/show/NCT02717442 (first received 23 March 2016).
Borghei 2016 {published data only}
-
- Borghei P, Sadeghian E, Hasanzadeh F, Emami H. Intratympanic dexamethasone delivery versus placebo in intractable Meniere disease. Academic Journal of Surgery 2016;3(3-4):58-62.
El Shafei 2020 {published data only}
-
- El Shafei RR, Qotb M. Comparison of the effect of three treatment interventions for the control of Meniere's disease: a randomized control trial. Egyptian Journal of Otolaryngology 2020;36(1):Article number 22. [DOI: 10.1186/s43163-020-00018-0] - DOI
Garduno‐Anaya 2005 {published data only}
-
- Garduno-Anaya MA, Couthino De Toledo H, Hinojosa-Gonzalez R, Pane-Pianese C, Rios-Castaneda LC. Dexamethasone inner ear perfusion by intratympanic injection in unilateral Ménière's disease: a two-year prospective, placebo-controlled, double-blind, randomized trial. Otolaryngology - Head and Neck Surgery 2005;133(2):285-94. [DOI: 10.1016/j.otohns.2005.05.010] - DOI - PubMed
-
- Garduno-Anaya MA, Pane-Pianese C, Couthino de Toledo H. Dexamethasone inner ear perfusion in unilateral Ménière's disease: four years of prospective evaluation. In: XVIII IFOS World Congress, Rome, Italy, 2005 June 25-30. 2005.
-
- Garduno-Anaya MA, Toledo de Couthino H, Hinojosa R, Pane-Pianese C, Castaneda CR. Intratympanic dexamethasone in unilateral Meniere's disease: a two-year prospective trial. Otolaryngology - Head and Neck Surgery 2003;129(2):P136. - PubMed
Lambert 2012 {published data only}
-
- Lambert PR, Nguyen S, Maxwell KS, Tucci DL, Lustig LR, Fletcher M, et al. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Ménière's disease. Otology & Neurotology 2012;33(7):1257-65. - PubMed
-
- NCT01412177. OTO-104 for the treatment of Meniere's disease (NCT01412177). https://clinicaltrials.gov/show/NCT01412177 (first received 9 August 2011).
Lambert 2016 {published data only}
-
- Lambert PR, Carey J, Mikulec AA, LeBel C, Otonomy Ménière's Study Group. Intratympanic sustained-exposure dexamethasone thermosensitive gel for symptoms of Ménière's disease: randomized phase 2b safety and efficacy trial. Otology & Neurotology 2016;37:1669-76. [DOI: DOI: 10.1097/MAO.0000000000001227] - PMC - PubMed
-
- Lambert PR, Carey JP, Mikulec A, Lebel CP. Ph2b efficacy and safety of intratympanic OTO-104 in Meniere's disease. Otolaryngology - Head and Neck Surgery 2015;153(1 Suppl 1):108.
-
- NCT01412177. OTO-104 for the treatment of Meniere's disease (NCT01412177). https://clinicaltrials.gov/show/NCT01412177 (first received 9 August 2011).
NCT02265393 {published data only}
-
- NCT02265393. A 6-month, prospective, randomized, multicenter, placebo-controlled safety study of OTO-104 given at 3-month intervals by intratympanic injection in subjects with unilateral Meniere's disease followed by a 6-month open-label extension (NCT02265393). https://clinicaltrials.gov/ct2/show/NCT02265393 (first received 15 October 2014).
-
- The safety of local application of dexamethasone (a corticosteroid) in the ear of people with Meniere's disease (EUCTR2014-001337-86-GB). http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-001337-86-GB .
NCT03664674 {published data only}
-
- A study to see of local application (in the ear) of dexamethasone (a corticosteroid) to people with Meniere's disease could help with their symptoms and if it is safe to use (EUCTR2018-001464-35-ES). https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... 2019.
-
- A study to see of local application (in the ear) of dexamethasone (a corticosteroid) to people with Meniere's disease could help with their symptoms and if it is safe to use (EUCTR2018-001464-35-GB). http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001464-35-GB 2018.
-
- NCT03664674. Phase 3 study of OTO-104 in subjects with unilateral Meniere's disease (NCT03664674). https://clinicaltrials.gov/show/nct03664674 (first received 10 September 2018).
-
- Otonomy announces top-line results for the phase 3 clinical trial of OTIVIDEX® in patients with Ménière's disease. https://investors.otonomy.com/news-releases/news-release-details/otonomy... 2021.
Ul Shamas 2017 {published data only}
-
- Ul Shamas I. Short term results of intra tympanic gentamicin and dexamethasone on hearing and tinnitus in Meniere's disease: a case control study. International Tinnitus Journal 2017;21(1):21-3. [DOI: DOI: 10.5935/0946-5448.20170005] - PubMed
References to studies excluded from this review
Alles 2006 {published data only}
-
- Alles MJ, Gaag MA, Stokroos RJ. Intratympanic steroid therapy for inner ear diseases, a review of the literature. European Archives of Oto-Rhino-Laryngology 2006;263(9):791-7. - PubMed
Conde 1965 {published data only}
-
- Conde Jahn F. Treatment of labyrinthine vertigo. Acta Oto-rino-laringologica Ibero-americana 1965;16(5):511-21. - PubMed
Dimitriadis 2017 {published data only}
-
- Dimitriadis PA, Zis P. Nocebo effect in Meniere's disease: a meta-analysis of placebo-controlled randomized controlled trials. Otology & Neurotology 2017;38(9):1370-5. - PubMed
Godlowski 1965 {published data only}
-
- Godlowski Z. Pathogenesis and management of Meniere's syndrome in terms of microcirculation. Pharmacologic decompression of the endolymphatic hydrops. Angiology 1965;16(11):644-50. - PubMed
Guo 2016 {published data only}
-
- Guo X, Wang Q, Li Y, Zhang Z. Clinical effect of prootic and opisthotic injection of dexamethasone and gentamicin in Meniere disease therapy. Tropical Journal of Pharmaceutical Research 2016;15(8):1781-6.
Hao 2022 {published data only}
Kitahara 2007 {published data only}
-
- Kitahara T, Okumura S-I, Kubo T, Kitahara M. Effects of intra-endolymphatic sac application of large doses of steroids for intractable Meniere's Disease: a randomized controlled trial. In: 26th Politzer Society Annual Meeting. Cleveland, Ohio, USA, 2007 October 13-16. 2007:Abstract No. 106.
Kitahara 2008 {published data only}
-
- Kitahara T, Kubo T, Okumura S, Kitahara M. Effects of endolymphatic sac drainage with steroids for intractable Meniere's disease: a long-term follow-up and randomized controlled study. Laryngoscope 2008;118(5):854-61. - PubMed
Maksoud 2015 {published data only}
-
- Maksoud AA, Hassan DM, Nafie Y, Saadb A. Intratympanic dexamethasone injection in Meniere's disease. Egyptian Journal of Otolaryngology 2015;31(2):128-34.
NCT02768662 {published data only}
-
- NCT02768662. A 6-month extension study of OTO-104 in Meniere's disease. https://ClinicalTrials.gov/show/NCT02768662 (first received 11 May 2016).
Patel 2017 {published data only}
Phillips 2011 {published data only}
Richards 1971 {published data only}
-
- Richards SH. Meniere's disease. Practitioner 1971;207(242):759-66. - PubMed
Sakata 1988 {published data only}
-
- Sakata E, Itoh Y, Teramoto K. Treatment of Morbus Meniere - transtympanic infusion of Lidocain, a corticosteroid solution, into the tympanic cavity. HNO 1988;36(9):386.
Silverstein 1998 {published data only}
-
- Silverstein H, Isaacson JE, Olds MJ, Rowan PT, Rosenberg S. Dexamethasone inner ear perfusion for the treatment of Meniere's disease: a prospective, randomized, double-blind, crossover trial. American Journal of Otology 1998;19(2):196-201. - PubMed
Syed 2015 {published data only}
-
- Syed MI, Ilan O, Nassar J, Rutka JA. Intratympanic therapy in Meniere's syndrome or disease: up to date evidence for clinical practice. Clinical Otolaryngology 2015;40(6):682-90. - PubMed
Thabet 2008 {published data only}
-
- Thabet ES, Kamel Y, Rizk N. Outcome and quality of life after intratympanic therapy for control of Meniere's disease. Benha Medical Journal 2008;25(2):297-8.
Additional references
AAO‐HNS 1995
-
- Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere's Disease. Otolaryngology – Head and Neck Surgery 1995;113(3):181-5. - PubMed
AAOO 1972
-
- Committee on Hearing and Equilibrium. Meniere's disease: criteria for diagnosis and evaluation of therapy for reporting. Transactions of the American Academy of Ophthalmology and Otolaryngology 1972;76:1462-4. - PubMed
Ahmadzai 2020
Albu 2016
-
- Albu S, Nagy A, Doros C, Marceanu L, Cozma S, Musat G, et al. Treatment of Meniere's disease with intratympanic dexamethazone plus high dosage of betahistine. American Journal of Otolaryngology - Head and Neck Medicine and Surgery 2016;37:225-30. - PubMed
Baloh 2001
-
- Baloh RW. Prosper Ménière and his disease. Archives of Neurology 2001;58:1151-6. - PubMed
Banks 2012
-
- Banks C, McGinness S, Harvey R, Sacks R. Is allergy related to Meniere's disease? Current Allergy and Asthma Reports 2012;12:255-60. - PubMed
Bruderer 2017
-
- Bruderer SG, Bodmer D, Stohler NA, Jick SS, Meier CR. Population-based study on the epidemiology of Ménière's disease. Audiology & Neuro-otology 2017;22(2):74-82. - PubMed
Cao 2019
-
- Cao Z, Yue F, Huang W, Rajenderkumar D, Zhao F . Different medications for the treatment of Meniere's disease by intratympanic injection: a systematic review and network meta-analysis. Clinical Otolaryngology 2019;44(4):619-27. - PubMed
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
Chiarella 2015
Chuang‐Chuang 2017
-
- Chuang-Chuang A, Baeza MA . Are intratympanic corticosteroids effective for Meniere's disease? [Son efectivos los corticoides intratimpanicos en la enfermedad de Meniere? ]. Medwave 2017;17:e6863. - PubMed
Devantier 2019
-
- Devantier L, Djurhuus BD, Hougaard DD, Handel MN, Guldfred FL, Schmidt JH, et al. Intratympanic steroid for Meniere's disease: a systematic review. Otology & Neurotology 2019;40(6):806-12. - PubMed
Doyle 2004
-
- Doyle KJ, Bauch C, Battista R, Beatty C, Hughes GB, Mason J, et al. Intratympanic steroid treatment: a review. Otology & Neurotology 2004;25(6):1034-9. - PubMed
Farhood 2016
-
- Farhood Z, Lambert PR. The physiologic role of corticosteroids in Ménière's disease. American Journal of Otolaryngology - Head and Neck Surgery 2016;37:455-8. - PubMed
Gacek 2009
-
- Gacek RR. Ménière's disease is a viral neuropathy. ORL; Journal for Oto-rhino-laryngology and Its Related Specialties 2009;71(2):78-86. - PubMed
Gates 2004
-
- Gates GA, Green JD, Tucci DL, Telian SA. The effects of transtympanic micropressure treatment in people with unilateral Meniere's disease. Archives of Otolaryngology - Head and Neck Surgery 2004;130(6):718-25. - PubMed
Greco 2012
-
- Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, Vincentiis M. Meniere's disease might be an autoimmune condition? Autoimmunity Reviews 2012;11(10):731-8. - PubMed
Hallpike 1938
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Handbook 2021
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Harcourt 2014
-
- Harcourt J, Barraclough K, Bronstein AM. Meniere's disease. BMJ 2014;349:g6544. - PubMed
Harris 2010
-
- Harris JP, Alexander TH. Current-day prevalence of Ménière’s syndrome. Audiology & Neuro-otology 2010;15(5):318-22. - PubMed
Honrubia 1996
-
- Honrubia V, Bell TS, Harris MR, Baloh RQ, Fisher LM. Quantitative evaluation of dizziness characteristics and impact on quality of life. American Journal of Otology 1996;17:595-602. - PubMed
Hu 2009
-
- Hu A, Parnes LS . Intratympanic steroids for inner ear disorders: a review. Audiology & Neuro-Otology 2009;14(6):373-82. - PubMed
Huppert 2010
Jacobsen 1990
-
- Jacobsen GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of Otolaryngology - Head & Neck Surgery 1990;116(4):424-7. - PubMed
Jacobsen 1998
-
- Jacobsen GP, Calder JH. A screening version of the Dizziness Handicap Inventory. American Journal of Otology 1998;19(6):804-8. - PubMed
Lopez‐Escamez 2015
-
- Lopez-Escamez JA, Carey J, Chung W, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière’s disease. Journal of Vestibular Research 2015;25:1-7. - PubMed
Meikle 2012
-
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. The Tinnitus Functional Index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing 2012;33(2):153-76. - PubMed
Murphy 1999
-
- Murphy MP, Gates GA. Measuring the effects of Menieres Disease: results of the Patient-Oriented Severity Index (MD POSI) Version 1. Annals of Otology, Rhinology and Laryngology 1999;108(4):331-7. - PubMed
Newman 1996
-
- Newman CW, Jacobsen GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology--Head and Neck Surgery 1996;122(2):143-8. - PubMed
Parnes 1999
-
- Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope 1999;109(S91):1-17. - PubMed
Rarey 1996
-
- Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngology - Head and Neck Surgery 1996;115(1):38-41. - PubMed
Requena 2014
-
- Requena T, Espinosa-Sanchez JM, Cabrera S, Trinidad G, Soto-Varela A, Santos-Perez S, et al. Familial clustering and genetic heterogeneity in Meniere’s disease. Clinical Genetics 2014;85:245-52. - PubMed
RevMan 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Söderman 2002
-
- Söderman AH, Bagger-Sjöbäck D, Bergenius J, Langius A. Factors influencing quality of life in patients with Ménière's disease, identified by a multidimensional approach. Otology & Neurotology 2002;23(6):941-8. - PubMed
Tesio 1999
-
- Tesio L, Alpini D, Cesarani A, Perucca L. Short form of the Dizziness Handicap Inventory: construction and validation through Rasch analysis. American Journal of Physical Medicine and Rehabilitation 1999;78(3):233-41. - PubMed
Tyrrell 2016
Wallace 2017
Wan 2014
Webster 2021a
Webster 2021b
Xu 2021
-
- Xu C, Furuya-Kanamori L, Zorzela L, Lin L, Vohra S. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. Journal of Clinical Epidemiology 2021;135:70-8. [DOI: ] - PubMed
Yamakawa 1938
-
- Yamakawa K. Hearing organ of a patient who showed Meniere's symptoms [Japanese]. Journal of the Otolaryngological Society of Japan 1938;44:2310-2.
Yardley 1992a
-
- Yardley L, Putman J. Quantitative analysis of factors contributing to handicap and distress in vertiginous patients: a questionnaire study. Clinical Otolaryngology 1992;17:231-6. - PubMed
Yardley 1992b
-
- Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. Journal of Psychometric Research 1992;36(8):731-41. - PubMed
Yardley 1998
Zeman 2011
-
- Zeman F, Koller M, Figueiredo R, Aazevedo A, Rates M, Coelho C, et al. Tinnitus Handicap Inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngology - Head and Neck Surgery 2011;145(2):282-7. [DOI: ] - PubMed
Zeng 2021
-
- Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RSC, Santesso N, Traversy G, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. Journal of Clinical Epidemiology 2021;137:163-75. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

