Dual Nickel Photocatalysis for O-Aryl Carbamate Synthesis from Carbon Dioxide
- PMID: 36848485
- PMCID: PMC10028690
- DOI: 10.1021/acs.joc.3c00023
Dual Nickel Photocatalysis for O-Aryl Carbamate Synthesis from Carbon Dioxide
Abstract
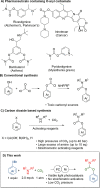
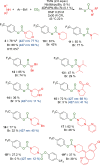
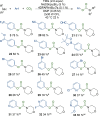
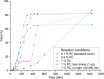
We report the use of dual nickel photocatalysis in the synthesis of O-aryl carbamates from aryl iodides or bromides, amines, and carbon dioxide. The reaction proceeded in visible light, at ambient carbon dioxide pressure, and without stoichiometric activating reagents. Mechanistic analysis is consistent with a Ni(I-III) cycle, where the active species is generated by the photocatalyst. The rate-limiting steps were the photocatalyst-mediated reduction of Ni(II) to Ni(I) and subsequent oxidative addition of the aryl halide. The physical properties of the photocatalyst were critical for promoting formation of O-aryl carbamates over various byproducts. Nine new phthalonitrile photocatalysts were synthesized, which exhibited properties that were vital to achieve high selectivity and activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Slocombe R. J.; Hardy E. E.; Saunders J. H.; Jenkins R. L. Phosgene Derivatives. The Preparation of Isocyanates, Carbamyl Chlorides and Cyanuric Acid. J. Am. Chem. Soc. 1950, 72 (5), 1888–1891. 10.1021/ja01161a009. - DOI
- Watson R. B.; Butler T. W.; DeForest J. C. Preparation of Carbamates, Esters, Amides, and Unsymmetrical Ureas via Brønsted Acid-Activated N-Acyl Imidazoliums. Org. Process Res, Dev. 2021, 25 (3), 500–506. 10.1021/acs.oprd.0c00445. - DOI
-
- Niemi T.; Repo T. Antibiotics from Carbon Dioxide: Sustainable Pathways to Pharmaceutically Relevant Cyclic Carbamates. Eur. J. Org. Chem. 2019, 2019 (6), 1180–1188. 10.1002/ejoc.201801598. - DOI
- Sakakura T.; Choi J. C.; Yasuda H. Transformation of carbon dioxide. Chem. Rev. 2007, 107 (6), 2365–2387. 10.1021/cr068357u. - DOI - PubMed
- Wang L.; Qi C.; Xiong W.; Jiang H. Recent advances in fixation of CO2 into organic carbamates through multicomponent reaction strategies. Chin. J. Catal. 2022, 43 (7), 1598–1617. 10.1016/S1872-2067(21)64029-9. - DOI
- Ye J. H.; Ju T.; Huang H.; Liao L. L.; Yu D. G. Radical Carboxylative Cyclizations and Carboxylations with CO(2). Acc. Chem. Res. 2021, 54 (10), 2518–2531. 10.1021/acs.accounts.1c00135. - DOI - PubMed
- Cai B.; Cheo H. W.; Liu T.; Wu J. Light-Promoted Organic Transformations Utilizing Carbon-Based Gas Molecules as Feedstocks. Angew. Chem., Int. Ed. 2021, 60 (35), 18950–18980. 10.1002/anie.202010710. - DOI - PubMed
- Riemer D.; Hirapara P.; Das S. Chemoselective Synthesis of Carbamates using CO2 as Carbon Source. ChemSusChem 2016, 9 (15), 1916–1920. 10.1002/cssc.201600521. - DOI - PubMed
-
- Farshbaf S.; Fekri L. Z.; Nikpassand M.; Mohammadi R.; Vessally E. Dehydrative condensation of β-aminoalcohols with CO2: An environmentally benign access to 2-oxazolidinone derivatives. J. CO2 Util. 2018, 25, 194–204. 10.1016/j.jcou.2018.03.020. - DOI
- Foo S. W.; Takada Y.; Yamazaki Y.; Saito S. Dehydrative synthesis of chiral oxazolidinones catalyzed by alkali metal carbonates under low pressure of CO2. Tetrahedron Lett. 2013, 54 (35), 4717–4720. 10.1016/j.tetlet.2013.06.100. - DOI
- Takada Y.; Foo S. W.; Yamazaki Y.; Saito S. Catalytic fluoride triggers dehydrative oxazolidinone synthesis from CO2. RSC Adv. 2014, 4 (92), 50851–50857. 10.1039/C4RA09609F. - DOI
- Tamura M.; Honda M.; Noro K.; Nakagawa Y.; Tomishige K. Heterogeneous CeO2-catalyzed selective synthesis of cyclic carbamates from CO2 and aminoalcohols in acetonitrile solvent. J. Catal. 2013, 305, 191–203. 10.1016/j.jcat.2013.05.013. - DOI
- Schilling W.; Das S. Transition Metal-Free Synthesis of Carbamates Using CO2 as the Carbon Source. ChemSusChem 2020, 13 (23), 6246–6258. 10.1002/cssc.202002073. - DOI - PubMed
- Zhang Z.; Ye J. H.; Wu D. S.; Zhou Y. Q.; Yu D. G. Synthesis of Oxazolidin-2-ones from Unsaturated Amines with CO(2) by Using Homogeneous Catalysis. Chem. Asian. J. 2018, 13 (17), 2292–2306. 10.1002/asia.201800672. - DOI - PubMed
- Aomchad V.; Cristòfol À; Della Monica F.; Limburg B.; D’Elia V.; Kleij A. W. Recent progress in the catalytic transformation of carbon dioxide into biosourced organic carbonates. Green Chem. 2021, 23 (3), 1077–1113. 10.1039/D0GC03824E. - DOI
LinkOut - more resources
Full Text Sources

