Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria
- PMID: 36848639
- PMCID: PMC10241857
- DOI: 10.1182/bloodadvances.2022009129
Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria
Abstract
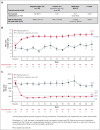
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disease characterized by complement-mediated hemolysis. Pegcetacoplan is the first C3-targeted therapy approved for adults with PNH (United States), adults with PNH with inadequate response or intolerance to a C5 inhibitor (Australia), and adults with anemia despite C5-targeted therapy for ≥3 months (European Union). PRINCE was a phase 3, randomized, multicenter, open-label, controlled study to evaluate the efficacy and safety of pegcetacoplan vs control (supportive care only; eg, blood transfusions, corticosteroids, and supplements) in complement inhibitor-naive patients with PNH. Eligible adults receiving supportive care only for PNH were randomly assigned and stratified based on their number of transfusions (<4 or ≥4) 12 months before screening. Patients received pegcetacoplan 1080 mg subcutaneously twice weekly or continued supportive care (control) for 26 weeks. Coprimary end points were hemoglobin stabilization (avoidance of >1-g/dL decrease in hemoglobin levels without transfusions) from baseline through week 26 and lactate dehydrogenase (LDH) change at week 26. Overall, 53 patients received pegcetacoplan (n = 35) or control (n = 18). Pegcetacoplan was superior to control for hemoglobin stabilization (pegcetacoplan, 85.7%; control, 0; difference, 73.1%; 95% confidence interval [CI], 57.2-89.0; P < .0001) and change from baseline in LDH (least square mean change: pegcetacoplan, -1870.5 U/L; control, -400.1 U/L; difference, -1470.4 U/L; 95% CI, -2113.4 to -827.3; P < .0001). Pegcetacoplan was well tolerated. No pegcetacoplan-related adverse events were serious, and no new safety signals were observed. Pegcetacoplan rapidly and significantly stabilized hemoglobin and reduced LDH in complement inhibitor-naive patients and had a favorable safety profile. This trial was registered at www.clinicaltrials.gov as NCT04085601.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.S.M.W. has received consulting fees, honoraria, research funding, and speaker’s bureau fees from Alexion Pharmaceuticals Inc and F. Hoffmann-La Roche Ltd. and research funding and speaker’s bureau fees from Apellis Pharmaceuticals, Inc. D.G.-A. has received speaking and advising roles from Teva, Roche, Amgen, Bristol Myers Squibb, Takeda, Janssen, AbbVie, and Astellas. M.A.-A. is an employee of Apellis Pharmaceuticals Inc. T.A., J.S., P.D., C.F., and F.G. are employees and current equity holders of the publicly traded company Apellis Pharmaceuticals Inc. P.A. reports former consultancy with Apellis Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Figures

References
-
- Peacock-Young B, Macrae FL, Newton DJ, Hill A, Ariëns RAS. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica. 2018;103(1):9–17. - PubMed
-
- Hill A, Sapsford RJ, Scally A, et al. Under-recognized complications in patients with paroxysmal nocturnal haemoglobinuria: raised pulmonary pressure and reduced right ventricular function. Br J Haematol. 2012;158(3):409–414. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

