Monitoring Temporal Changes in SARS-CoV-2 Spike Antibody Levels and Variant-Specific Risk for Infection, Dominican Republic, March 2021-August 2022
- PMID: 36848869
- PMCID: PMC10045678
- DOI: 10.3201/eid2904.221628
Monitoring Temporal Changes in SARS-CoV-2 Spike Antibody Levels and Variant-Specific Risk for Infection, Dominican Republic, March 2021-August 2022
Abstract
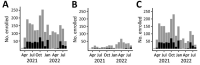
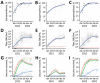
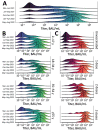
To assess changes in SARS-CoV-2 spike binding antibody prevalence in the Dominican Republic and implications for immunologic protection against variants of concern, we prospectively enrolled 2,300 patients with undifferentiated febrile illnesses in a study during March 2021-August 2022. We tested serum samples for spike antibodies and tested nasopharyngeal samples for acute SARS-CoV-2 infection using a reverse transcription PCR nucleic acid amplification test. Geometric mean spike antibody titers increased from 6.6 (95% CI 5.1-8.7) binding antibody units (BAU)/mL during March-June 2021 to 1,332 (95% CI 1,055-1,682) BAU/mL during May-August 2022. Multivariable binomial odds ratios for acute infection were 0.55 (95% CI 0.40-0.74), 0.38 (95% CI 0.27-0.55), and 0.27 (95% CI 0.18-0.40) for the second, third, and fourth versus the first anti-spike quartile; findings were similar by viral strain. Combining serologic and virologic screening might enable monitoring of discrete population immunologic markers and their implications for emergent variant transmission.
Keywords: COVID-19; Dominican Republic; SARS-CoV-2; coronavirus disease; respiratory infections; severe acute respiratory syndrome coronavirus 2; spike antibody; vaccine-preventable diseases; viruses; zoonoses.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

