Immunopotentiation effects of apigenin on NK cell proliferation and killing pancreatic cancer cells
- PMID: 36848930
- PMCID: PMC9974612
- DOI: 10.1177/03946320231161174
Immunopotentiation effects of apigenin on NK cell proliferation and killing pancreatic cancer cells
Abstract
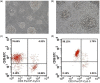
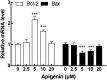
Apigenin is a kind of flavonoid with many beneficial biological effects. It not only has direct cytotoxicity to tumor cells, but also can boost the antitumor effect of immune cells by modulating immune system. The purpose of this study was to investigate the proliferation of NK cells treated with apigenin and its cytotoxicity to pancreatic cancer cells in vitro, and explore its potential molecular mechanism. In this study, the effect of apigenin on NK cell proliferation and killing pancreatic cancer cells were measured by CCK-8 assay. Perforin, granzyme B (Gran B), CD107a, and NKG2D expressions of NK cells induced with apigenin were detected by flow cytometry (FCM). The mRNA expression of Bcl-2, Bax and protein expression of Bcl-2, Bax, p-ERK, and p-JNK in NK cells were evaluated by qRT-PCR and western blotting analysis, respectively. The results showed that appropriate concentration of apigenin could significantly promote the proliferation of NK cells in vitro and enhance the killing activity of NK cells against pancreatic cancer cells. The expressions of surface antigen NKG2D and intracellular antigen perforin and Gran B of NK cells were upregulated after treating with apigenin. Bcl-2 mRNA expression was increased, while Bax mRNA expression was decreased. Similarly, the expression of Bcl-2, p-JNK, and p-ERK protein was upregulated, and the expression of Bax protein was downregulated. The molecular mechanism of the immunopotentiation effects of apigenin may be that it up-regulates Bcl-2 and down-regulates Bax expression at the gene and protein levels to facilitate NK cell proliferation, and up-regulates the expression of perforin, Gran B, and NKG2D through the activation of JNK and ERK pathways to enhance NK cell cytotoxicity.
Keywords: Apigenin; NK cell; immunopotentiation; killing activity; pancreatic cancer.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Siegel RL, Miller KD, Fuchs HE, et al. (2021) Cancer statistics, 2021. CA A Cancer J Clin 71(1): 7–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

