Ubiquitin-like conjugation by bacterial cGAS enhances anti-phage defence
- PMID: 36848932
- PMCID: PMC10097602
- DOI: 10.1038/s41586-023-05862-7
Ubiquitin-like conjugation by bacterial cGAS enhances anti-phage defence
Abstract
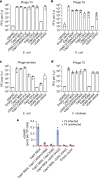
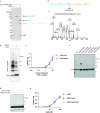
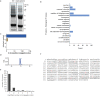
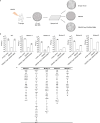
cGAS is an evolutionarily conserved enzyme that has a pivotal role in immune defence against infection1-3. In vertebrate animals, cGAS is activated by DNA to produce cyclic GMP-AMP (cGAMP)4,5, which leads to the expression of antimicrobial genes6,7. In bacteria, cyclic dinucleotide (CDN)-based anti-phage signalling systems (CBASS) have been discovered8-11. These systems are composed of cGAS-like enzymes and various effector proteins that kill bacteria on phage infection, thereby stopping phage spread. Of the CBASS systems reported, approximately 39% contain Cap2 and Cap3, which encode proteins with homology to ubiquitin conjugating (E1/E2) and deconjugating enzymes, respectively8,12. Although these proteins are required to prevent infection of some bacteriophages8, the mechanism by which the enzymatic activities exert an anti-phage effect is unknown. Here we show that Cap2 forms a thioester bond with the C-terminal glycine of cGAS and promotes conjugation of cGAS to target proteins in a process that resembles ubiquitin conjugation. The covalent conjugation of cGAS increases the production of cGAMP. Using a genetic screen, we found that the phage protein Vs.4 antagonized cGAS signalling by binding tightly to cGAMP (dissociation constant of approximately 30 nM) and sequestering it. A crystal structure of Vs.4 bound to cGAMP showed that Vs.4 formed a hexamer that was bound to three molecules of cGAMP. These results reveal a ubiquitin-like conjugation mechanism that regulates cGAS activity in bacteria and illustrates an arms race between bacteria and viruses through controlling CDN levels.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

