Regulation of astrocyte lipid metabolism and ApoE secretionby the microglial oxysterol, 25-hydroxycholesterol
- PMID: 36849076
- PMCID: PMC10060115
- DOI: 10.1016/j.jlr.2023.100350
Regulation of astrocyte lipid metabolism and ApoE secretionby the microglial oxysterol, 25-hydroxycholesterol
Abstract
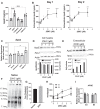
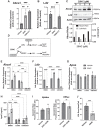
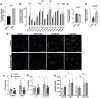
Neuroinflammation, a major hallmark of Alzheimer's disease and several other neurological and psychiatric disorders, is often associated with dysregulated cholesterol metabolism. Relative to homeostatic microglia, activated microglia express higher levels of Ch25h, an enzyme that hydroxylates cholesterol to produce 25-hydroxycholesterol (25HC). 25HC is an oxysterol with interesting immune roles stemming from its ability to regulate cholesterol metabolism. Since astrocytes synthesize cholesterol in the brain and transport it to other cells via ApoE-containing lipoproteins, we hypothesized that secreted 25HC from microglia may influence lipid metabolism as well as extracellular ApoE derived from astrocytes. Here, we show that astrocytes take up externally added 25HC and respond with altered lipid metabolism. Extracellular levels of ApoE lipoprotein particles increased after treatment of astrocytes with 25HC without an increase in Apoe mRNA expression. In mouse astrocytes-expressing human ApoE3 or ApoE4, 25HC promoted extracellular ApoE3 better than ApoE4. Increased extracellular ApoE was due to elevated efflux from increased Abca1 expression via LXRs as well as decreased lipoprotein reuptake from suppressed Ldlr expression via inhibition of SREBP. 25HC also suppressed expression of Srebf2, but not Srebf1, leading to reduced cholesterol synthesis in astrocytes without affecting fatty acid levels. We further show that 25HC promoted the activity of sterol-o-acyl transferase that led to a doubling of the amount of cholesteryl esters and their concomitant storage in lipid droplets. Our results demonstrate an important role for 25HC in regulating astrocyte lipid metabolism.
Keywords: 25-hydroxycholesterol; Alzheimer disease; apolipoprotein E; astrocyte; cholesterol metabolism; microglia; neuroinflammation; oxysterols.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest S. M. P. is a cofounder, board member and shareholder of Sage Therapeutics, Voyager Therapeutics and Alnylam Pharmaceuticals. He is also a board member and shareholder of Karuna Pharmaceuticals and a Venture Partner at Third Rock Ventures. D. M. H. cofounded and is on the scientific advisory board of C2N Diagnostics. D. M. H. is on the scientific advisory board of Denali and Cajal Neuroscience and consults for Genentech and Alector.
Figures




References
-
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., et al. A unique microglia type associated with restricting development of Alzheimer’s Disease. Cell. 2017;169:1276–1290.e17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

