Age-related methylation changes in the human sperm epigenome
- PMID: 36849136
- PMCID: PMC10042684
- DOI: 10.18632/aging.204546
Age-related methylation changes in the human sperm epigenome
Abstract
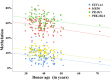
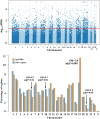
Advanced paternal age is associated with increased risks for reproductive and offspring medical problems. Accumulating evidence suggests age-related changes in the sperm epigenome as one underlying mechanism. Using reduced representation bisulfite sequencing on 73 sperm samples of males attending a fertility center, we identified 1,162 (74%) regions which were significantly (FDR-adjusted) hypomethylated and 403 regions (26%) being hypermethylated with age. There were no significant correlations with paternal BMI, semen quality, or ART outcome. The majority (1,152 of 1,565; 74%) of age-related differentially methylated regions (ageDMRs) were located within genic regions, including 1,002 genes with symbols. Hypomethylated ageDMRs were closer to transcription start sites than hypermethylated DMRs, half of which reside in gene-distal regions. In this and conceptually related genome-wide studies, so far 2,355 genes have been reported with significant sperm ageDMRs, however most (90%) of them in only one study. The 241 genes which have been replicated at least once showed significant functional enrichments in 41 biological processes associated with development and the nervous system and in 10 cellular components associated with synapses and neurons. This supports the hypothesis that paternal age effects on the sperm methylome affect offspring behaviour and neurodevelopment. It is interesting to note that sperm ageDMRs were not randomly distributed throughout the human genome; chromosome 19 showed a highly significant twofold enrichment with sperm ageDMRs. Although the high gene density and CpG content have been conserved, the orthologous marmoset chromosome 22 did not appear to exhibit an increased regulatory potential by age-related DNA methylation changes.
Keywords: ART outcome; DNA methylation; human sperm epigenome; male germ cells; paternal age effect.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

