Heartburn relief with bicarbonate-rich mineral water: results of the randomised, placebo-controlled phase-III trial STOMACH STILL
- PMID: 36849190
- PMCID: PMC9972411
- DOI: 10.1136/bmjgast-2022-001048
Heartburn relief with bicarbonate-rich mineral water: results of the randomised, placebo-controlled phase-III trial STOMACH STILL
Abstract
Objective: We assessed whether the bicarbonate-rich mineral water Staatl. Fachingen STILL is superior over conventional mineral water in relieving heartburn.
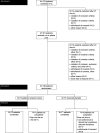
Design: Multicentre, double-blind, randomised, placebo-controlled trial STOMACH STILL in adult patients with frequent heartburn episodes since ≥6 months and without moderate/severe reflux oesophagitis. Patients drank 1.5 L/day verum or placebo over the course of the day for 6 weeks. Primary endpoint was the percentage of patients with reduction of ≥5 points in the Reflux Disease Questionnaire (RDQ) score for 'heartburn'. Secondary endpoints included symptom reduction (RDQ), health-related quality of life (HRQOL, Quality of Life in Reflux and Dyspepsia (QOLRAD)), intake of rescue medication and safety/tolerability.
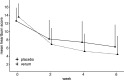
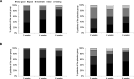
Results: Of 148 randomised patients (verum: n=73, placebo: n=75), 143 completed the trial. Responder rates were 84.72% in the verum and 63.51% in the placebo group (p=0.0035, number needed to treat=5). Symptoms improved under verum compared with placebo for the dimension 'heartburn' (p=0.0003) and the RDQ total score (p=0.0050). HRQOL improvements under verum compared with placebo were reported for 3 of 5 QOLRAD domains, that is, 'food/drink problems' (p=0.0125), 'emotional distress' (p=0.0147) and 'vitality' (p=0.0393). Mean intake of rescue medication decreased from 0.73 tablets/day at baseline to 0.47 tablets/day in week 6 in the verum group, whereas in the placebo group it remained constant during the trial. Only three patients had treatment-related adverse events (verum: n=1, placebo: n=2).
Conclusion: STOMACH STILL is the first controlled clinical trial demonstrating superiority of a mineral water over placebo in relieving heartburn, accompanied by an improved HRQOL.
Trial registration number: EudraCT 2017-001100-30.
Keywords: acid-related diseases; anti-reflux therapy; dyspepsia; gastric diseases; gastroesophageal reflux disease.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: During conduct of the trial and preparation of the manuscript, JL received honoraria for consultancy from Fachingen Heil- und Mineralbrunnen GmbH. MA and JW were employees of SocraTec R&D GmbH. R-SW was an employee of SocraTec R&D GmbH and SocraMetrics GmbH. HW was an employee of Fachingen Heil- und Mineralbrunnen GmbH. BS was cofounder and managing director of SocraTec R&D GmbH and SocraMetrics GmbH.
Figures
References
-
- Wilkinson JM, Halland M. Esophageal motility disorders. Am Fam Physician 2020;102:291–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
