Ribosomes in RNA Granules Are Stalled on mRNA Sequences That Are Consensus Sites for FMRP Association
- PMID: 36849416
- PMCID: PMC10082463
- DOI: 10.1523/JNEUROSCI.1002-22.2023
Ribosomes in RNA Granules Are Stalled on mRNA Sequences That Are Consensus Sites for FMRP Association
Abstract
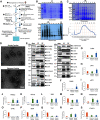
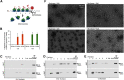
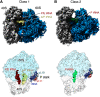
Local translation in neurons is partly mediated by the reactivation of stalled polysomes. Stalled polysomes may be enriched within the granule fraction, defined as the pellet of sucrose gradients used to separate polysomes from monosomes. The mechanism of how elongating ribosomes are reversibly stalled and unstalled on mRNAs is still unclear. In the present study, we characterize the ribosomes in the granule fraction using immunoblotting, cryogenic electron microscopy (cryo-EM), and ribosome profiling. We find that this fraction, isolated from 5-d-old rat brains of both sexes, is enriched in proteins implicated in stalled polysome function, such as the fragile X mental retardation protein (FMRP) and Up-frameshift mutation 1 homologue. Cryo-EM analysis of ribosomes in this fraction indicates they are stalled, mainly in the hybrid state. Ribosome profiling of this fraction reveals (1) an enrichment for footprint reads of mRNAs that interact with FMRPs and are associated with stalled polysomes, (2) an abundance of footprint reads derived from mRNAs of cytoskeletal proteins implicated in neuronal development, and (3) increased ribosome occupancy on mRNAs encoding RNA binding proteins. Compared with those usually found in ribosome profiling studies, the footprint reads were longer and were mapped to reproducible peaks in the mRNAs. These peaks were enriched in motifs previously associated with mRNAs cross-linked to FMRP in vivo, independently linking the ribosomes in the granule fraction to the ribosomes associated with FMRP in the cell. The data supports a model in which specific sequences in mRNAs act to stall ribosomes during translation elongation in neurons.SIGNIFICANCE STATEMENT Neurons send mRNAs to synapses in RNA granules, where they are not translated until an appropriate stimulus is given. Here, we characterize a granule fraction obtained from sucrose gradients and show that polysomes in this fraction are stalled on consensus sequences in a specific state of translational arrest with extended ribosome-protected fragments. This finding greatly increases our understanding of how neurons use specialized mechanisms to regulate translation and suggests that many studies on neuronal translation may need to be re-evaluated to include the large fraction of neuronal polysomes found in the pellet of sucrose gradients used to isolate polysomes.
Keywords: cryo-EM; elongation; neuroscience; protein synthesis; ribosome profiling; stalled ribosome.
Copyright © 2023 the authors.
Figures


Similar articles
-
FMRP Control of Ribosome Translocation Promotes Chromatin Modifications and Alternative Splicing of Neuronal Genes Linked to Autism.Cell Rep. 2020 Mar 31;30(13):4459-4472.e6. doi: 10.1016/j.celrep.2020.02.076. Cell Rep. 2020. PMID: 32234480 Free PMC article.
-
In vitro Analysis of Stalled Ribosomes Using Puromycin Incorporation.Bio Protoc. 2023 Aug 20;13(16):e4744. doi: 10.21769/BioProtoc.4744. eCollection 2023 Aug 20. Bio Protoc. 2023. PMID: 37638299 Free PMC article.
-
Tracking the Fragile X Mental Retardation Protein in a Highly Ordered Neuronal RiboNucleoParticles Population: A Link between Stalled Polyribosomes and RNA Granules.PLoS Genet. 2016 Jul 27;12(7):e1006192. doi: 10.1371/journal.pgen.1006192. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27462983 Free PMC article.
-
mRNPs, polysomes or granules: FMRP in neuronal protein synthesis.Curr Opin Neurobiol. 2006 Jun;16(3):265-9. doi: 10.1016/j.conb.2006.05.010. Epub 2006 May 16. Curr Opin Neurobiol. 2006. PMID: 16707258 Review.
-
Dial tm for rescue: tmRNA engages ribosomes stalled on defective mRNAs.Curr Opin Struct Biol. 2004 Feb;14(1):58-65. doi: 10.1016/j.sbi.2004.01.010. Curr Opin Struct Biol. 2004. PMID: 15102450 Review.
Cited by
-
FMRP-mediated spatial regulation of physiologic NMD targets in neuronal cells.Genome Biol. 2024 Jan 23;25(1):31. doi: 10.1186/s13059-023-03146-x. Genome Biol. 2024. PMID: 38263082 Free PMC article. Review.
-
Opto-CLIP reveals dynamic FMRP regulation of mRNAs upon CA1 neuronal activation.bioRxiv [Preprint]. 2025 Jan 15:2024.08.13.607210. doi: 10.1101/2024.08.13.607210. bioRxiv. 2025. PMID: 39185177 Free PMC article. Preprint.
-
Puromycin reveals a distinct conformation of neuronal ribosomes.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2306993121. doi: 10.1073/pnas.2306993121. Epub 2024 Feb 5. Proc Natl Acad Sci U S A. 2024. PMID: 38315848 Free PMC article.
-
Construction destruction: Contribution of dyregulated proteostasis to neurodevelopmental disorders.Curr Opin Neurobiol. 2025 Feb;90:102934. doi: 10.1016/j.conb.2024.102934. Epub 2024 Nov 28. Curr Opin Neurobiol. 2025. PMID: 39612590 Review.
-
Dysregulated ribosome quality control in human diseases.FEBS J. 2025 Mar;292(5):936-959. doi: 10.1111/febs.17217. Epub 2024 Jul 1. FEBS J. 2025. PMID: 38949989 Free PMC article. Review.
References
-
- Amorim IS, Kedia S, Kouloulia S, Simbriger K, Gantois I, Jafarnejad SM, Li Y, Kampaite A, Pooters T, Romanò N, Gkogkas CG (2018) Loss of eIF4E phosphorylation engenders depression-like behaviors via selective mRNA translation. J Neurosci 38:2118–2133. 10.1523/JNEUROSCI.2673-17.2018 - DOI - PMC - PubMed
-
- Anadolu MN, Sossin WS (2021) Focusing on mRNA granules and stalled polysomes amidst diverse mechanisms underlying mRNA transport, mRNA storage and local translation. In: Oxford handbook of neuronal protein synthesis. (Sossin W, ed), pp. 239–268. NewYork: Oxford UP.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
