The double-edged functions of necroptosis
- PMID: 36849530
- PMCID: PMC9969390
- DOI: 10.1038/s41419-023-05691-6
The double-edged functions of necroptosis
Abstract
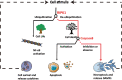
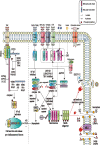
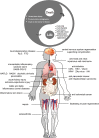
Necroptosis refers to a regulated form of cell death induced by a variety of stimuli. Although it has been implicated in the pathogenesis of many diseases, there is evidence to support that necroptosis is not purely a detrimental process. We propose that necroptosis is a "double-edged sword" in terms of physiology and pathology. On the one hand, necroptosis can trigger an uncontrolled inflammatory cascade response, resulting in severe tissue injury, disease chronicity, and even tumor progression. On the other hand, necroptosis functions as a host defense mechanism, exerting antipathogenic and antitumor effects through its powerful pro-inflammatory properties. Moreover, necroptosis plays an important role during both development and regeneration. Misestimation of the multifaceted features of necroptosis may influence the development of therapeutic approaches targeting necroptosis. In this review, we summarize current knowledge of the pathways involved in necroptosis as well as five important steps that determine its occurrence. The dual role of necroptosis in a variety of physiological and pathological conditions is also highlighted. Future studies and the development of therapeutic strategies targeting necroptosis should fully consider the complicated properties of this type of regulated cell death.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

