State-of-the-art evidence in the treatment of systemic sclerosis
- PMID: 36849541
- PMCID: PMC9970138
- DOI: 10.1038/s41584-023-00909-5
State-of-the-art evidence in the treatment of systemic sclerosis
Abstract
Systemic sclerosis (SSc) is a rare autoimmune connective tissue disease with multi-organ involvement, fibrosis and vasculopathy. Treatment in SSc, including early diffuse cutaneous SSc (dcSSc) and the use of organ-specific therapies, has improved, as evident from randomized clinical trials. Treatments for early dcSSc include immunosuppressive agents such as mycophenolate mofetil, methotrexate, cyclophosphamide, rituximab and tocilizumab. Patients with rapidly progressive early dcSSc might be eligible for autologous haematopoietic stem cell transplantation, which can improve survival. Morbidity from interstitial lung disease and pulmonary arterial hypertension is improving with the use of proven therapies. Mycophenolate mofetil has surpassed cyclophosphamide as the initial treatment for SSc-interstitial lung disease. Nintedanib and possibly perfinidone can be considered in SSc pulmonary fibrosis. Pulmonary arterial hypertension is frequently treated with initial combination therapy (for example, with phosphodiesterase 5 inhibitors and endothelin receptor antagonists) and, if necessary, the addition of a prostacyclin analogue. Raynaud phenomenon and digital ulcers are treated with dihydropyridine calcium channel blockers (especially nifedipine), then phosphodiesterase 5 inhibitors or intravenous iloprost. Bosentan can reduce the development of new digital ulcers. Trial data for other manifestations are mostly lacking. Research is needed to develop targeted and highly effective treatments, best practices for organ-specific screening and early intervention, and sensitive outcome measurements.
© 2023. Springer Nature Limited.
Conflict of interest statement
J.E.P. declares that she has research grants from AbbVie, Bayer, BI, BMS, Frensenius Kabi, Lilly, Mallinckrodt Pharmaceuticals, Merck, Roche, Seattle Genetics; that she has consulted for AbbVie, Amgen, BI, BMS, Celltrion, EMERALD, Frensenius Kabi, Galapagos, Gilead, Janssen, Lilly, Mallinckrodt Pharmaceuticals, Medexus, Merck, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Roche, Sandoz, Samsung, Sanofi, Sobi, Teva, Viatris; and that she has been a speaker or attended an advisory board for AbbVie, Amgen, BI, BMS, Frensenius Kabi, Galapagos, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Sandoz, Sanofi, UCB. C.P.D. declares that he has received consultancy or speaker fees from GlaxoSmithKline, Roche, Boehringer Ingelheim, Sanofi-Aventis, Galapagos, Inventiva, Corbus, Acceleron, Horizon, Gesynta; and that he has received research grants to his institution from GlaxoSmithKline, ARXX Therapeutics, Servier and Horizon Therapeutics. S.R.J. declares that he has been a site investigator for clinical trials sponsored by Bayer, Boehringer Ingelheim, Corbus, GlaxoSmithKline; that he has served on advisory boards for Boehringer Ingelheim, Corbus and Ikaria; and that he has been supported by the Oscar and Eleonor Markovitz Scleroderma Research Fund and the Gurmej Kaur Dhanda Scleroderma Research Fund. A.F.-C. declares that he has received grant support from the Scleroderma Society of Ontario and honoraria from Actelion, Bayer, Boehringer-Ingelheim. M.H. declares that she has received research grants from Boehringer Ingelheim and Bristol Myers Squibb and that she has participated in advisory boards for Boehringer Ingelheim, Alexion and Mallinckrodt. T.N. declares no competing interests.
Figures

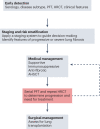
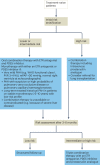

Comment in
-
Is classifying SSc-ILD drugs as either immunosuppressive or anti-fibrotic misleading?Nat Rev Rheumatol. 2023 Oct;19(10):675. doi: 10.1038/s41584-023-01013-4. Nat Rev Rheumatol. 2023. PMID: 37605003 No abstract available.
-
Reply to: Is classifying SSc-ILD drugs as either immunosuppressive or anti-fibrotic misleading?Nat Rev Rheumatol. 2023 Oct;19(10):676. doi: 10.1038/s41584-023-01014-3. Nat Rev Rheumatol. 2023. PMID: 37605004 No abstract available.
References
-
- LeRoy EC, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 1988;15:202–205. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

