Structure, folding and flexibility of co-transcriptional RNA origami
- PMID: 36849548
- PMCID: PMC10566746
- DOI: 10.1038/s41565-023-01321-6
Structure, folding and flexibility of co-transcriptional RNA origami
Abstract
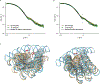
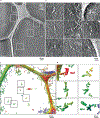
RNA origami is a method for designing RNA nanostructures that can self-assemble through co-transcriptional folding with applications in nanomedicine and synthetic biology. However, to advance the method further, an improved understanding of RNA structural properties and folding principles is required. Here we use cryogenic electron microscopy to study RNA origami sheets and bundles at sub-nanometre resolution revealing structural parameters of kissing-loop and crossover motifs, which are used to improve designs. In RNA bundle designs, we discover a kinetic folding trap that forms during folding and is only released after 10 h. Exploration of the conformational landscape of several RNA designs reveal the flexibility of helices and structural motifs. Finally, sheets and bundles are combined to construct a multidomain satellite shape, which is characterized by individual-particle cryo-electron tomography to reveal the domain flexibility. Together, the study provides a structural basis for future improvements to the design cycle of genetically encoded RNA nanodevices.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



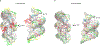







References
-
- Ohno H, Akamine S & Saito H RNA nanostructures and scaffolds for biotechnology applications. Curr. Opin. Biotechnol 58, 53–61 (2019). - PubMed
-
- Kim J & Franco E RNA nanotechnology in synthetic biology. Curr. Opin. Biotechnol 63, 135–141 (2020). - PubMed
-
- Geary C, Rothemund PW & Andersen ES A single-stranded architecture for cotranscriptional folding of RNA nanostructures. Science 345, 799–804 (2014). - PubMed
-
- Høiberg HC, Sparvath SM, Andersen VL, Kjems J & Andersen ES An RNA origami octahedron with intrinsic siRNAs for potent gene knockdown. Biotechnol. J 14, 1700634 (2018). - PubMed

