Cell-extracellular matrix mechanotransduction in 3D
- PMID: 36849594
- PMCID: PMC10656994
- DOI: 10.1038/s41580-023-00583-1
Cell-extracellular matrix mechanotransduction in 3D
Abstract
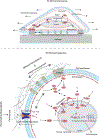
Mechanical properties of extracellular matrices (ECMs) regulate essential cell behaviours, including differentiation, migration and proliferation, through mechanotransduction. Studies of cell-ECM mechanotransduction have largely focused on cells cultured in 2D, on top of elastic substrates with a range of stiffnesses. However, cells often interact with ECMs in vivo in a 3D context, and cell-ECM interactions and mechanisms of mechanotransduction in 3D can differ from those in 2D. The ECM exhibits various structural features as well as complex mechanical properties. In 3D, mechanical confinement by the surrounding ECM restricts changes in cell volume and cell shape but allows cells to generate force on the matrix by extending protrusions and regulating cell volume as well as through actomyosin-based contractility. Furthermore, cell-matrix interactions are dynamic owing to matrix remodelling. Accordingly, ECM stiffness, viscoelasticity and degradability often play a critical role in regulating cell behaviours in 3D. Mechanisms of 3D mechanotransduction include traditional integrin-mediated pathways that sense mechanical properties and more recently described mechanosensitive ion channel-mediated pathways that sense 3D confinement, with both converging on the nucleus for downstream control of transcription and phenotype. Mechanotransduction is involved in tissues from development to cancer and is being increasingly harnessed towards mechanotherapy. Here we discuss recent progress in our understanding of cell-ECM mechanotransduction in 3D.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

