The artificial sweetener erythritol and cardiovascular event risk
- PMID: 36849732
- PMCID: PMC10334259
- DOI: 10.1038/s41591-023-02223-9
The artificial sweetener erythritol and cardiovascular event risk
Abstract
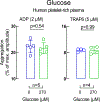
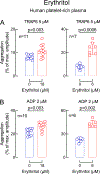
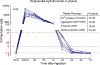
Artificial sweeteners are widely used sugar substitutes, but little is known about their long-term effects on cardiometabolic disease risks. Here we examined the commonly used sugar substitute erythritol and atherothrombotic disease risk. In initial untargeted metabolomics studies in patients undergoing cardiac risk assessment (n = 1,157; discovery cohort, NCT00590200 ), circulating levels of multiple polyol sweeteners, especially erythritol, were associated with incident (3 year) risk for major adverse cardiovascular events (MACE; includes death or nonfatal myocardial infarction or stroke). Subsequent targeted metabolomics analyses in independent US (n = 2,149, NCT00590200 ) and European (n = 833, DRKS00020915 ) validation cohorts of stable patients undergoing elective cardiac evaluation confirmed this association (fourth versus first quartile adjusted hazard ratio (95% confidence interval), 1.80 (1.18-2.77) and 2.21 (1.20-4.07), respectively). At physiological levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo. Finally, in a prospective pilot intervention study ( NCT04731363 ), erythritol ingestion in healthy volunteers (n = 8) induced marked and sustained (>2 d) increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies. Our findings reveal that erythritol is both associated with incident MACE risk and fosters enhanced thrombosis. Studies assessing the long-term safety of erythritol are warranted.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Dr. Hazen reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, being a paid consultant formerly for Procter & Gamble and currently with Zehna Therapeutics. He also reports having received research funds from Procter & Gamble, Zehna Therapeutics and Roche Diagnostics, and being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, a wholly owned subsidiary of Quest Diagnostics, Procter & Gamble and Zehna therapeutics. Dr. Tang reports being a consultant for Sequana Medical A.G., Owkin Inc, Relypsa Inc, and PreCardiac Inc, having received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation - all unrelated to the subject and contents of this paper. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


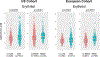







Comment in
-
Artificial sweetener linked to higher CVD risk.Nat Rev Cardiol. 2023 May;20(5):283. doi: 10.1038/s41569-023-00855-5. Nat Rev Cardiol. 2023. PMID: 36918524 No abstract available.
-
Is the Nonnutritive Sweetener Erythritol or Its Circulating Metabolite a Risk Factor for Cardiovascular Events?Clin Chem. 2023 Oct 3;69(10):1098-1100. doi: 10.1093/clinchem/hvad069. Clin Chem. 2023. PMID: 37473406 No abstract available.
References
-
- Buerge IJ, Buser HR, Kahle M, Müller MD & Poiger T Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ Sci Technol 43, 4381–4385 (2009). - PubMed
-
- Roberts A The safety and regulatory process for low calorie sweeteners in the United States. Physiol Behav 164, 439–444 (2016). - PubMed
-
- Mortensen A Sweeteners permitted in the European Union: safety aspects. Scandinavian Journal of Food and Nutrition 50, 104–116 (2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

