Asia-Pacific multicentre randomized trial of laparoscopic versus open major hepatectomy for hepatocellular carcinoma (AP-LAPO trial)
- PMID: 36849753
- PMCID: PMC9970867
- DOI: 10.1093/bjsopen/zrac166
Asia-Pacific multicentre randomized trial of laparoscopic versus open major hepatectomy for hepatocellular carcinoma (AP-LAPO trial)
Abstract
Background: Hepatocellular carcinoma is the sixth most common malignancy in the world. Major hepatectomy (resection of greater than or equal to three liver segments) is needed if a tumour is large or close to major blood vessels. Despite low mortality, open major hepatectomy is associated with high rates of tumour recurrence that limits survival. Laparoscopic major hepatectomy has been proposed as an alternative approach with potential oncological benefits. This study compares laparoscopic major hepatectomy with open major hepatectomy for hepatocellular carcinoma in a randomized trial.
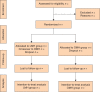
Methods: The Asia-Pacific multicentre randomized trial of laparoscopic versus open major hepatectomy for hepatocellular carcinoma (AP-LAPO trial) is an open-labelled multicentre randomized trial to be conducted in five centres in the Asia-Pacific region. The study will test the hypothesis that laparoscopic major hepatectomy for hepatocellular carcinoma is associated with less tumour recurrence and better survival compared with open major hepatectomy; the primary outcome being 2-year recurrence-free survival. Secondary outcomes include hospital mortality, postoperative complications according to the Clavien-Dindo classification, time to functional recovery, quality of life, long-term survival, and postoperative serum surgical stress-related cytokines.
Results and conclusion: The AP-LAPO trial will determine whether laparoscopic major hepatectomy offers oncological benefits to patients with hepatocellular carcinoma compared with open major hepatectomy.
Registration number: NCT04852211 (http://www.clinicaltrials.gov) registered on 21 April 2021.
Protocol version: AP-LAPO trial version 01 (1 December 2021).
© The Author(s) 2023. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 - PubMed
-
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236 - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LRet al. . AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380 - PubMed
-
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer Tet al. . Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv238–iv255 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous