Janus-type emission from a cyclometalated iron(III) complex
- PMID: 36849804
- PMCID: PMC10070185
- DOI: 10.1038/s41557-023-01137-w
Janus-type emission from a cyclometalated iron(III) complex
Abstract
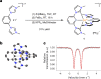
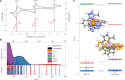
Although iron is a dream candidate to substitute noble metals in photoactive complexes, realization of emissive and photoactive iron compounds is demanding due to the fast deactivation of their charge-transfer states. Emissive iron compounds are scarce and dual emission has not been observed before. Here we report the FeIII complex [Fe(ImP)2][PF6] (HImP = 1,1'-(1,3-phenylene)bis(3-methyl-1-imidazol-2-ylidene)), showing a Janus-type dual emission from ligand-to-metal charge transfer (LMCT)- and metal-to-ligand charge transfer (MLCT)-dominated states. This behaviour is achieved by a ligand design that combines four N-heterocyclic carbenes with two cyclometalating aryl units. The low-lying π* levels of the cyclometalating units lead to energetically accessible MLCT states that cannot evolve into LMCT states. With a lifetime of 4.6 ns, the strongly reducing and oxidizing MLCT-dominated state can initiate electron transfer reactions, which could constitute a basis for future applications of iron in photoredox catalysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Monat JE, McCusker JK. Femtosecond excited-state dynamics of an iron(ii) polypyridyl solar cell sensitizer model. J. Am. Chem. Soc. 2000;122:4092–4097. doi: 10.1021/ja992436o. - DOI
-
- Zimmer P, et al. Towards noble-metal-free dyads: ground and excited state tuning by a cobalt dimethylglyoxime motif connected to an iron N-heterocyclic carbene photosensitizer. Eur. J. Inorg. Chem. 2018;2018:5203–5214. doi: 10.1002/ejic.201800946. - DOI
Grants and funding
- BA 4467/7-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- LO 714/11-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- KU 952/12-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- HE 2778/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ME 1313/15-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

