Extracellular vesicles derived from mesenchymal stem cells - a novel therapeutic tool in infectious diseases
- PMID: 36849892
- PMCID: PMC9970864
- DOI: 10.1186/s41232-023-00266-6
Extracellular vesicles derived from mesenchymal stem cells - a novel therapeutic tool in infectious diseases
Abstract
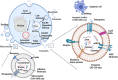
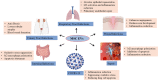
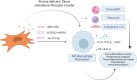
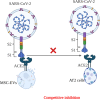
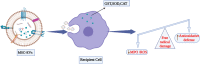
Extracellular vesicles (EVs) are nano-sized lipid-bilayer encapsulated vesicles produced by the cells. These EVs are released into the surrounding space by almost all cell types. The EVs help in intercellular communication via their payloads which contain various proteins, lipids, and nucleic acids generated from the donor cells and allow for synergistic responses in surrounding cells. In recent years, EVs have been increasingly important in treating infectious diseases, including respiratory tract infections, urinary tract infections, wound infections, sepsis, and intestinal infections. Studies have confirmed the therapeutic value of mesenchymal stem cell-derived EVs (MSC-EVs) for treating infectious diseases to eliminate the pathogen, modulate the resistance, and restore tissue damage in infectious diseases. This can be achieved by producing antimicrobial substances, inhibiting pathogen multiplication, and activating macrophage phagocytic activity. Pathogen compounds can be diffused by inserting them into EVs produced and secreted by host cells or by secreting them as microbial cells producing EVs carrying signalling molecules and DNA shielding infected pathogens from immune attack. EVs play a key role in infectious pathogenesis and hold great promise for developing innovative treatments. In this review, we discuss the role of MSC-EVs in treating various infectious diseases.
Keywords: Exosomes; Extracellular vesicles; Mesenchymal stem cells; Stem cell therapy; Wound infections.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

