A deep learning approach for fully automated cardiac shape modeling in tetralogy of Fallot
- PMID: 36849960
- PMCID: PMC9969707
- DOI: 10.1186/s12968-023-00924-1
A deep learning approach for fully automated cardiac shape modeling in tetralogy of Fallot
Abstract
Background: Cardiac shape modeling is a useful computational tool that has provided quantitative insights into the mechanisms underlying dysfunction in heart disease. The manual input and time required to make cardiac shape models, however, limits their clinical utility. Here we present an end-to-end pipeline that uses deep learning for automated view classification, slice selection, phase selection, anatomical landmark localization, and myocardial image segmentation for the automated generation of three-dimensional, biventricular shape models. With this approach, we aim to make cardiac shape modeling a more robust and broadly applicable tool that has processing times consistent with clinical workflows.
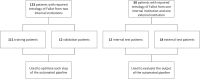
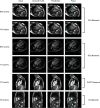
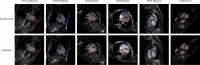
Methods: Cardiovascular magnetic resonance (CMR) images from a cohort of 123 patients with repaired tetralogy of Fallot (rTOF) from two internal sites were used to train and validate each step in the automated pipeline. The complete automated pipeline was tested using CMR images from a cohort of 12 rTOF patients from an internal site and 18 rTOF patients from an external site. Manually and automatically generated shape models from the test set were compared using Euclidean projection distances, global ventricular measurements, and atlas-based shape mode scores.
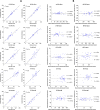
Results: The mean absolute error (MAE) between manually and automatically generated shape models in the test set was similar to the voxel resolution of the original CMR images for end-diastolic models (MAE = 1.9 ± 0.5 mm) and end-systolic models (MAE = 2.1 ± 0.7 mm). Global ventricular measurements computed from automated models were in good agreement with those computed from manual models. The average mean absolute difference in shape mode Z-score between manually and automatically generated models was 0.5 standard deviations for the first 20 modes of a reference statistical shape atlas.
Conclusions: Using deep learning, accurate three-dimensional, biventricular shape models can be reliably created. This fully automated end-to-end approach dramatically reduces the manual input required to create shape models, thereby enabling the rapid analysis of large-scale datasets and the potential to deploy statistical atlas-based analyses in point-of-care clinical settings. Training data and networks are available from cardiacatlas.org.
Keywords: Cardiovascular magnetic resonance (CMR); Congenital heart disease; Deep learning; Image segmentation; Shape modeling.
© 2023. The Author(s).
Conflict of interest statement
ADM and JHO are co-founders of, scientific advisors to, and equity holders in Insilicomed, Inc. ADM is also a co-founder of and scientific advisor to Vektor Medical, Inc. Some of their research grants have been identified for conflict-of-interest management. The authors are required to disclose these relationships in publications acknowledging the grant support; however, the findings reported in this study did not involve the companies in any way and have no relationship with the business activities or scientific interests of either company. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict-of-interest policies. The rest of the authors do not have any conflict-of-interest.
Figures